추천 제품
Grade
analytical standard
Quality Level
제품 라인
PESTANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
agriculture
environmental
형식
neat
SMILES string
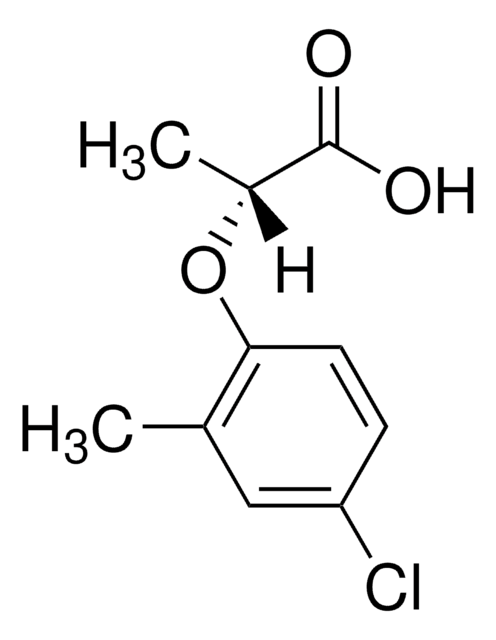
CC(Oc1ccc(Cl)cc1C)C(O)=O
InChI
1S/C10H11ClO3/c1-6-5-8(11)3-4-9(6)14-7(2)10(12)13/h3-5,7H,1-2H3,(H,12,13)
InChI key
WNTGYJSOUMFZEP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
법적 정보
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Pablo J Maid et al.
Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases, 24(4), 177-182 (2017-12-13)
Biologic agents may induce immune responses that could impact drug action. The aims of this study were to assess antidrug antibodies (ADAs) in patients with rheumatoid arthritis (RA) from Argentina treated with etanercept, adalimumab, or infliximab at a single visit
Régis Peffault de Latour et al.
British journal of haematology, 191(3), 476-485 (2020-05-26)
Ravulizumab, a novel long-acting complement component 5 (C5) inhibitor administered every 8 weeks (q8w), was non-inferior to eculizumab for all efficacy outcomes in two randomised, open-label, phase 3 trials in C5 inhibitor-naïve (Study 301) and eculizumab-experienced (Study 302) adult patients
Yutaka Osuga et al.
Obstetrics and gynecology, 133(3), 423-433 (2019-02-12)
To investigate the noninferiority of relugolix compared with leuprorelin acetate in reducing heavy menstrual bleeding associated with uterine leiomyomas. In a double-blind, double-dummy trial, premenopausal women with uterine leiomyomas and heavy menstrual bleeding defined as a pictorial blood loss assessment
Julien Taieb et al.
Drugs, 79(13), 1375-1394 (2019-07-28)
The approval of targeted therapies for metastatic colorectal cancer (mCRC) has led to important improvements in patient outcomes. However, it is still necessary to increase individualisation of treatments based on tumour genetic profiles to optimise efficacy, while minimising toxicity. As
Jeffrey Vietri et al.
Human vaccines & immunotherapeutics, 16(1), 161-168 (2019-07-26)
The CDC Advisory Committee on Immunization Practices (ACIP) recommended immunization with the recently licensed 13-valent pneumococcal conjugate vaccine (PCV13) for high-risk (immunocompromised) adults aged ≥19 years in 2012. This was in addition to the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Data
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.