모든 사진(1)
About This Item
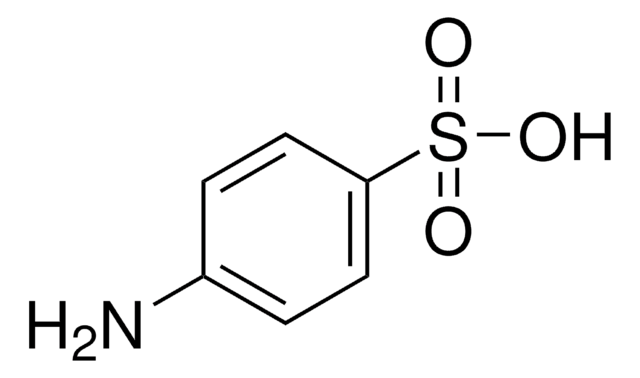
Linear Formula:
H2NC6H4SO2NH2
CAS Number:
Molecular Weight:
172.20
Beilstein:
511852
EC Number:
MDL number:
UNSPSC 코드:
41116107
eCl@ss:
39093202
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
Agency
EPA 1694
제품 라인
VETRANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
164-166 °C (lit.)
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
응용 분야
clinical testing
형식
neat
동작 모드
DNA synthesis | interferes
enzyme | inhibits
SMILES string
Nc1ccc(cc1)S(N)(=O)=O
InChI
1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10)
InChI key
FDDDEECHVMSUSB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: sulfonamide
Sulfanilamide is an antibacterial drug.
애플리케이션
It was used in a study to demonstrate photodecomposition in aqueous solution of cutaneous photosensitizing agents with the help of spin traps 5, 5-dimethyl-1-pyrroline-1-oxide.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
법적 정보
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Daniela Vullo et al.
Bioorganic & medicinal chemistry letters, 20(7), 2178-2182 (2010-03-10)
A beta-carbonic anhydrase (CA, EC 4.2.1.1) from the bacterial pathogen Brucella suis, bsCA 1, has been cloned, purified characterized kinetically and for inhibition with a series of water soluble glycosylated sulfanilamides. bsCA 1 has appreciable activity as catalyst for the
Edward E Knaus et al.
Bioorganic & medicinal chemistry letters, 21(19), 5892-5896 (2011-08-20)
A series of compounds incorporating regioisomeric phenylethynylbenzenesulfonamide moieties has been investigated for the inhibition of four human carbonic anhydrase (hCA, EC 4.2.1.1) isoforms, hCA I, II, IX and XII. Inhibition between the low nanomolar to the milliomolar range has been
Isao Nishimori et al.
Journal of medicinal chemistry, 48(24), 7860-7866 (2005-11-24)
A lately discovered carbonic anhydrase (hCA, EC 4.2.1.1), the mitochondrial hCA VB, was cloned, expressed, and purified. Kinetic parameters proved it to be 3.37 times more effective than hCA VA as a catalyst for the physiological reaction, with kcat =
Pascale Joseph et al.
Journal of medicinal chemistry, 53(5), 2277-2285 (2010-02-18)
A beta-carbonic anhydrase (CA, EC 4.2.1.1) from the bacterial pathogen Brucella suis, bsCA 1, has been cloned, purified, and characterized kinetically. bsCA 1 has appreciable activity as catalyst for the hydration of CO(2) to bicarbonate, with a k(cat) of 6.4
Isao Nishimori et al.
Journal of medicinal chemistry, 50(2), 381-388 (2007-01-19)
The secretory isozyme of human carbonic anhydrase (hCA, EC 4.2.1.1), hCA VI, has been cloned, expressed, and purified in a bacterial expression system. The kinetic parameters for the CO2 hydration reaction proved hCA VI to possess a kcat of 3.4
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.