모든 사진(4)
About This Item
실험식(Hill 표기법):
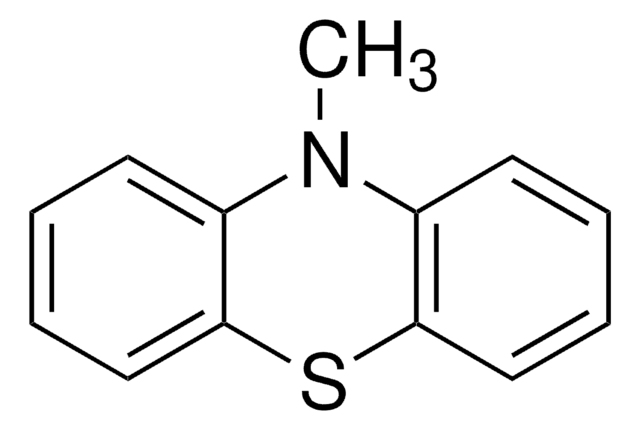
C12H9NS
CAS Number:
Molecular Weight:
199.27
Beilstein:
143237
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
bp:
371 °C (lit.)
추천 제품
일반 설명
The structure of phenothiazine is rigid, being tricyclic. It is known to alter dopamine (3,4-dihydroxyphenethylamine). Its use as an electron donor is based on its unique hole transporting ability, electron releasing nitrogen and sulfur heteroatoms and its non-planar structure leading to lower molecular aggregation.
애플리케이션
Phenothiazine finds uses in metal free organic dye sensitizers, dyes and antioxidants.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1 - STOT RE 2 Oral
표적 기관
Blood
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
A S Horn et al.
Proceedings of the National Academy of Sciences of the United States of America, 68(10), 2325-2328 (1971-10-01)
Phenothiazines and butyrophenones are known to alter dopamine (3,4-dihydroxyphenethylamine) metabolism in the brain in a fashion suggesting that they may block dopamine receptors. We observed, using Dreiding molecular models, that dopamine in its solid-state conformation is superimposable upon a portion
Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease
Carsten K, et al
Proceedings of the National Academy of Sciences of the USA, 98(17), 9834-9841 (2001)
Asif M, et al
Arabian Journal of Chemistry null
Photodegradation of trimeprazine triggered by self-photogenerated singlet molecular oxygen
Waseem A, et al
Journal of Saudi Chemical Society (2012)
Lavinia Baciu-Atudosie et al.
Bioorganic & medicinal chemistry letters, 22(22), 6896-6902 (2012-10-06)
A new family of protein farnesyltransferase inhibitors, based on a phenothiazine scaffold, was designed and synthesized. The biological evaluation of these products showed that compounds 28 and 30 were the most active, with protein farnesyltransferase inhibition potencies in the low
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.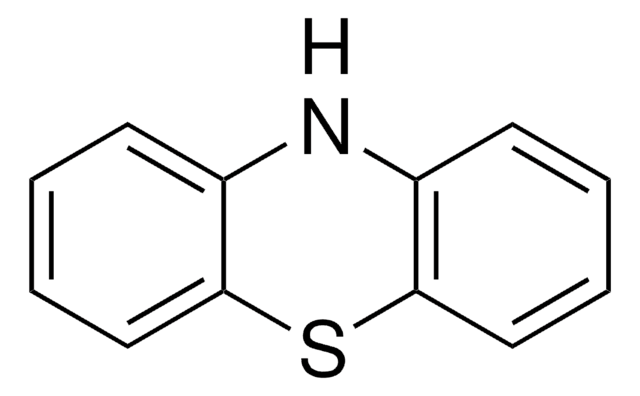








![5H-Dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/396/216/18f00414-a76e-46d7-90cf-820ad902e559/640/18f00414-a76e-46d7-90cf-820ad902e559.png)