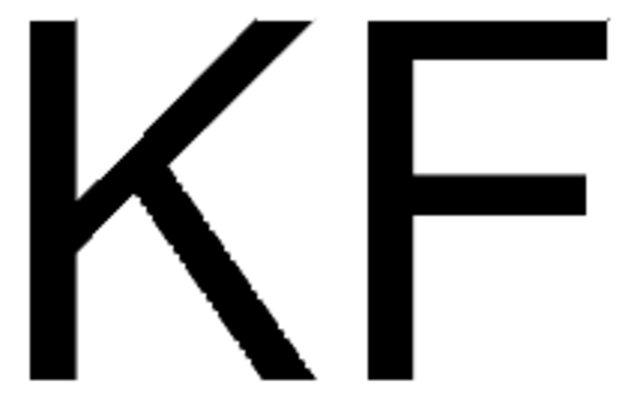모든 사진(1)
About This Item
실험식(Hill 표기법):
CsF
CAS Number:
Molecular Weight:
151.90
MDL number:
UNSPSC 코드:
12352100
추천 제품
Quality Level
형태
solid
구성
, 14-16 wt. % (loading of base)
SMILES string
[F-].[Cs+]
InChI
1S/Cs.FH/h;1H/q+1;/p-1
InChI key
XJHCXCQVJFPJIK-UHFFFAOYSA-M
일반 설명
Cesium fluoride is an inorganic compound known to be a source of fluoride ion and a catalyst in organic synthesis. It has been used in many organic reactions like 1,4−elimination, desilylation, transesterification, acylation, nucleophilic aromatic substitution, etherification, cross−coupling reactions and so on.
애플리케이션
Cesium fluoride can be used as:
A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
Preparation of building blocks for synthesis of fluoroallylic compounds
Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
For general uses, product is also available in powdered form (198323)
A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
Preparation of building blocks for synthesis of fluoroallylic compounds
Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
For general uses, product is also available in powdered form (198323)
특징 및 장점
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
기타 정보
High-Throughput Reaction Screening with Nanomoles of Solid Reagents Coated on Glass Beads
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Repr. 2 - STOT RE 2
표적 기관
Kidney,Adrenal gland
보충제 위험성
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Dong Wook Kim et al.
Journal of the American Chemical Society, 128(50), 16394-16397 (2006-12-15)
Aprotic solvents are usually preferred for the SN2 reactions, because nucleophilicity and hence SN2 reactivity are severely retarded by the influence of the partial positive charge of protic solvents. In this work, we introduce a remarkable effect of using tertiary
Shengcai Zheng et al.
Chemical communications (Cambridge, England), 47(24), 6969-6971 (2011-05-20)
One pot asymmetrical double allylations of sodium sulfide catalyzed by an iridium complex along with a combination of caesium fluoride and water in dichloromethane have been realized and the double allylation products with two C-S bond chiral centers were obtained
Timothy Noël et al.
Angewandte Chemie (International ed. in English), 50(38), 8900-8903 (2011-08-13)
[Image: see text] A flow process for Pd-catalyzed carbon fluorine bond formation is described. A microreactor using a packed-bed design allows for easy handling of large quantities of insoluble CsF with precise control over reaction times, efficient mixing, and the
Michael C Willis et al.
Chemical communications (Cambridge, England), (8)(8), 832-833 (2002-07-19)
Treatment of a benzyl substituted meso-ditriflate with boronic acids in the presence of palladium acetate, triphenylphosphine and caesium fluoride results in intermolecular Suzuki coupling followed by vinyl triflate-arene cyclisation to provide, in high yields, single regioisomers of tricyclic-carbocycles.
Takashi Okitsu et al.
Chemical communications (Cambridge, England), (47)(47), 6330-6332 (2008-12-03)
A highly efficient and rapid total synthesis of 9Z-retinoic acid was accomplished by caesium fluoride-promoted Stille coupling reaction; using a common building block, 9Z-retinoic acid analogues were also prepared by the same method without isomerisation of the Z-double bond.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.