PHR1024
Lactose (Monohydrate)
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
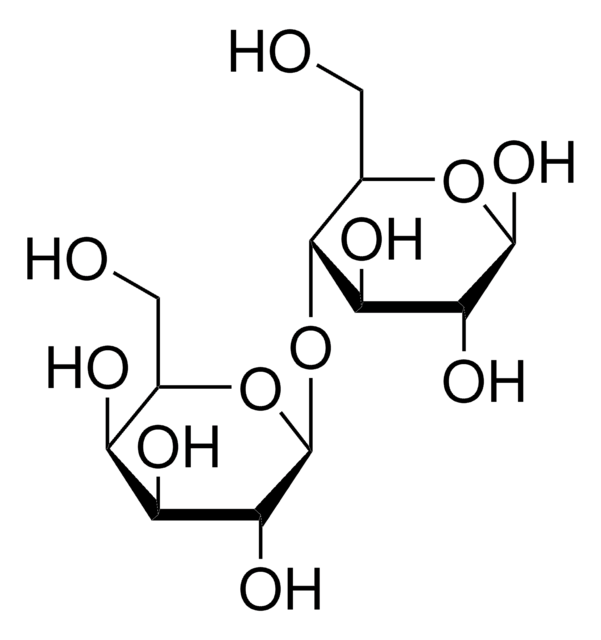
D-Lactose monohydrate, β-D-Gal-(1→4)-D-Glc, 4-O-β-D-Galactopyranosyl-D-glucose, Milk sugar
About This Item
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001750
traceable to USP 1356701
API family
lactose
CofA
current certificate can be downloaded
분석물 화학적 분류
oligosaccharides
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
cleaning products
cosmetics
detection
environmental
food and beverages
personal care
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
O.OC[C@@H](O)[C@@H](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)[C@H](O)[C@@H](O)C=O
InChI
1S/C12H22O11.H2O/c13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12;/h1,4-12,14-21H,2-3H2;1H2/t4-,5+,6+,7+,8-,9-,10+,11+,12-;/m0./s1
InChI key
HBDJFVFTHLOSDW-XBLONOLSSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
분석 메모
기타 정보
각주
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.