PHR1364
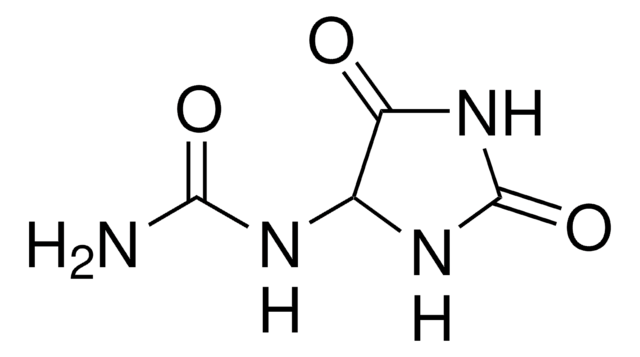
Allantoin
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
Allantoin, 5-Ureidohydantoin, Glyoxylic(acid) diureide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C4H6N4O3
CAS Number:
Molecular Weight:
158.12
Beilstein:
102364
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. A0349000
traceable to USP 1012939
API family
allantoin
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
230 °C (dec.) (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
NC(=O)NC1NC(=O)NC1=O
InChI
1S/C4H6N4O3/c5-3(10)6-1-2(9)8-4(11)7-1/h1H,(H3,5,6,10)(H2,7,8,9,11)
InChI key
POJWUDADGALRAB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Allantoin, the final catabolic product of purines in mammals, is a highly polar and amphoteric substance. Allantoin is mainly used for skin protection as the drug promotes cell proliferation and wound healing.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Allantoin may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
생화학적/생리학적 작용
Purine metabolite via the uric acid pathway. Uric acid also reacts with free radicals to produce allantoin, thus allantoin may be a useful biomarker for oxidative stress.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA1468 in the slot below. This is an example certificate only and may not be the lot that you receive.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Simultaneous quantification of allantoin and steroidal saponins in yam (Dioscorea spp.) powders
Maksimovic K, et al.
Journal of Applied Research on Medicinal and Aromatic Plants, 13(7), 100200-100200 (2019)
Quantification of allantoin in various Zea mays L. hybrids by RP-HPLC with UV detection
Maksimovic K, et al.
Pharmazie, 59(7), 524-527 (2004)
Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection
El Mubarak MAS, et al.
Journal of Chromatography A, 1322, 49-53 (2013)
X B Chen et al.
Journal of AOAC International, 79(3), 628-635 (1996-05-01)
Methods for determining allantoin in biological, cosmetic, and pharmaceutical samples are reviewed. The methods cover 3 main categories: spectrophotometry, alkalimetric titration, and chromatography (thin-layer and liquid). Comments are given on suitability for different sample types and on further development of
[Allantoin].
T Ogihara et al.
Nihon rinsho. Japanese journal of clinical medicine, 57 Suppl, 769-771 (2001-02-07)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.