Y0000155
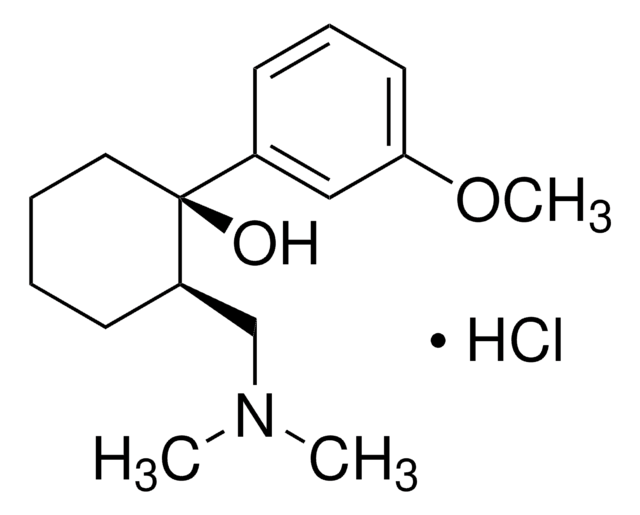
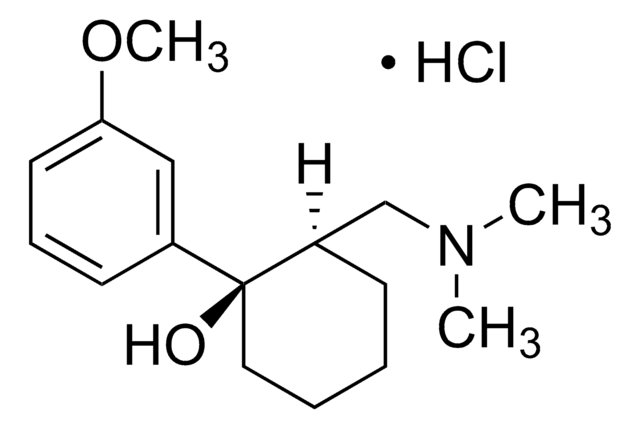
Tramadol hydrochloride
European Pharmacopoeia (EP) Reference Standard
동의어(들):
(±)-cis-2-(Dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C16H25NO2 · HCl
CAS Number:
Molecular Weight:
299.84
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
tramadol
제조업체/상표
EDQM
약물 제어
USDEA Schedule IV
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
Cl.COc1cccc(c1)[C@@]2(O)CCCC[C@@H]2CN(C)C
InChI
1S/C16H25NO2.ClH/c1-17(2)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)19-3;/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3;1H/t14-,16+;/m1./s1
InChI key
PPKXEPBICJTCRU-XMZRARIVSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Tramadol is a synthetic opiate agonist analgesic, widely used in patients suffering from moderate to severe chronic pain. It is considered safe when compared to other opioids. It contributes to the analgesic activity by blocking the nociceptive impulses at the spinal level, inhibiting the norepinephrine and serotonin reuptake.
Tramadol is a synthetic opiate agonist analgesic, widely used in patients suffering from moderate to severe chronic pain. It is considered safe when compared to other opioids. It contributes to the analgesic activity by blocking the nociceptive impulses at the spinal level, inhibiting the norepinephrine and serotonin reuptake.
애플리케이션
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Opioid analgesic; P450 substrate.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Application of HPLC for the simultaneous determination of aceclofenac, paracetamol and tramadol hydrochloride in pharmaceutical dosage form
Chandra P, et al.
Scientia Pharmaceutica, 80(2), 337-352 (2012)
The clinical use of tramadol hydrochloride
Bamigbade AT and Langford MR
Pain, 5(6), 155-182 (1998)
Analgesic oral efficacy of tramadol hydrochloride in postoperative pain
Sunshine A, et al.
Clinical Pharmacology and Therapeutics, 51(6), 740-746 (1992)
Kinetic spectrophotometric determination of tramadol hydrochloride in pharmaceutical formulation
Abdellatef EH
Journal of Pharmaceutical and Biomedical Analysis, 29(5), 835-842 (2002)
HPLC determination and validation of tramadol hydrochloride in capsules
Kartinasari FW, et al.
Journal of Liquid Chromatography and Related Technologies, 27(4), 737-744 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.