Y0000326
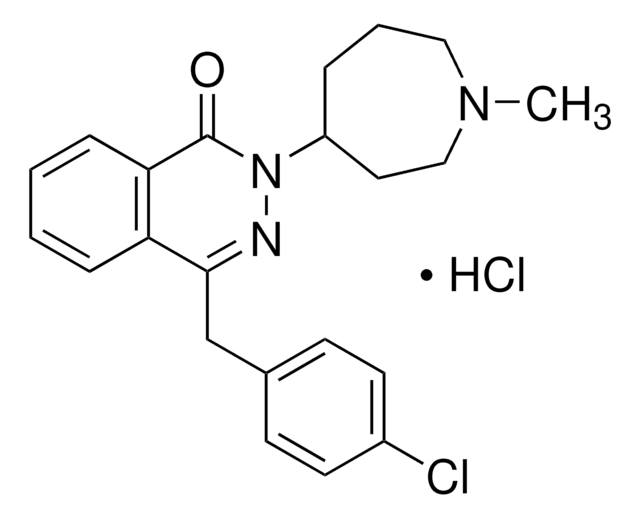
Azelastine hydrochloride
European Pharmacopoeia (EP) Reference Standard
동의어(들):
4-[(4-chlorophenyl)methyl]-2-(1-methylazepan-4-yl)phthalazin-1-one hydrochloride, Astelin, Optivar
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C22H24ClN3O · HCl
CAS Number:
Molecular Weight:
418.36
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
azelastine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
Cl.CN1CCCC(CC1)N2N=C(Cc3ccc(Cl)cc3)c4ccccc4C2=O
InChI
1S/C22H24ClN3O.ClH/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16;/h2-3,6-11,18H,4-5,12-15H2,1H3;1H
InChI key
YEJAJYAHJQIWNU-UHFFFAOYSA-N
유전자 정보
human ... HRH1(3269)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Azelastine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
H1 histamine receptor antagonist; NF-kB activator.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Jonathan A Bernstein
Current medical research and opinion, 23(10), 2441-2452 (2007-08-29)
Azelastine hydrochloride (Astelin) nasal spray 0.1% solution is a second-generation intranasal antihistamine available in the US for treatment of both seasonal allergic rhinitis (SAR) and nonallergic vasomotor rhinitis (VMR). Searches of journal articles including the title word 'azelastine' from 1979
Hartmut Derendorf et al.
British journal of clinical pharmacology, 74(1), 125-133 (2012-02-24)
• The topical second generation anti-histamine azelastine hydrochloride (AZE) and the potent corticosteroid fluticasone propionate (FP) are well established first-line treatments in allergic rhinitis (AR). • MP29-02, a novel intranasal AZE and FP formulation, has been shown to control AR
Daniel Baumann et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 80(1), 156-163 (2011-09-29)
For locally acting drugs, an extended residence time in the nasal cavity is desirable and related to a prolonged effect. We sought to develop a model for comparative determination of intranasal pharmacokinetics. We embedded human respiratory tissue into a solid
Warner W Carr et al.
Allergy and asthma proceedings, 33(6), 450-458 (2012-11-07)
Intranasal corticosteroids are considered the most effective therapy for moderate-to-severe seasonal allergic rhinitis (SAR) and recommended first line in guidelines. It is uncertain whether intranasal antihistamines have comparable efficacy. This study was designed to compare the efficacy of azelastine (AZE;
Alisa Rudnitskaya et al.
Analytica chimica acta, 770, 45-52 (2013-03-19)
The application of the potentiometric multisensor system (electronic tongue, ET) for quantification of the bitter taste of structurally diverse active pharmaceutical ingredients (API) is reported. The measurements were performed using a set of bitter substances that had been assessed by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.