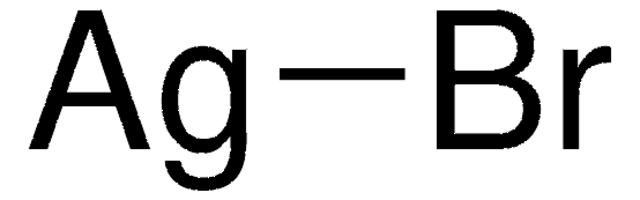추천 제품
일반 설명
Silver chloride (AgCl) is widely employed as a catalyst, photographic material, and ionic semiconductor material. High photosensitivity of AgCl makes it an important photocatalyst.
애플리케이션
Silver chloride can be used as a catalyst:
Silver chloride is also be used:
- In the preparation of 1,2,3-triazoles from alkynes and azides.
Silver chloride is also be used:
- As a precursor salt to synthesize silver nanoparticles.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Met. Corr. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Band structure of silver chloride and silver bromide.
Scop PM.
Physical Review, 139(3A), A934-A934 (1965)
Ionic conductivity of silver chloride single crystals.
Corish J and Jacobs PWM.
Journal of Physics and Chemistry of Solids, 33(7), 1799-1818 (1972)
A type of silver chloride electrode suitable for use in dilute solutions.
Brown AS.
Journal of the American Chemical Society, 56(3), 646-647 (1934)
Tomohisa Hayashi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 28(2), 127-133 (2012-02-11)
Electrochemical detection of sugar-related compounds was conducted using a boron-doped diamond (BDD) electrode as a detector for flow-injection analysis (FIA). Sugar-related compounds oxidize at high applied potentials, for which the BDD electrode is suitable for electrochemical measurements. Conditions for an
Alevtina Neyman et al.
Chemical communications (Cambridge, England), 48(16), 2207-2209 (2012-01-19)
"Out-of-pocket" association of Ag(+) to the tetradentate defect site of mono-vacant Keggin and Wells-Dawson polyoxometalate (POM) cluster-anions is used to direct the formation of water-soluble AgCl nanocubes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.