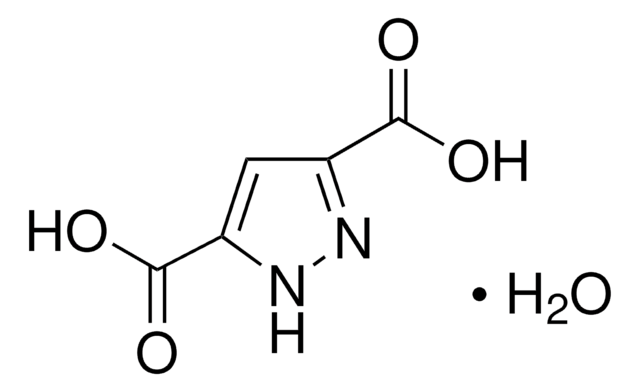
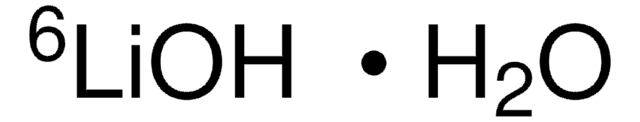추천 제품
일반 설명
Lithium hydroxide (LiOH) is an alkali metal hydroxide. Lithium chloride solution in water on electrolysis forms LiOH. In respiratory apparatus and submarines, it is utilized to uptake carbon dioxide. A study on the redox mechanism of titanium dioxide (TiO2) using cyclic voltammetry, X-ray diffraction, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) in the aqueous LiOH electrolyte has been reported.
애플리케이션
Lithium hydroxide may be used in the following processes:
- Synthesis of lithium-doped zinc oxide (ZnO) thin films.
- Preparation of lithium glyceroxide/hydroxide catalysts by reacting with glycerol.
- As a catalyst to generate unsaturated ketones via Michael addition of β-dicarbonyl compounds.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
G F Zhang et al.
Journal of animal science, 92(7), 2987-2995 (2014-05-08)
The objective of this experiment was to determine the net energy requirements for maintenance of growing and finishing pigs using regression models. Thirty-six growing (27.38 ± 2.24 kg) and 36 finishing (70.25 ± 2.61 kg) barrows were used and within
Lithium Hydroxide-Catalyzed Conjugate Addition of β-Dicarbonyl Compounds.
Bonadies F, et al.
ChemInform, 26(23) (1995)
Eagleson M.
Concise Encyclopedia Chemistry, 605-605 (1994)
Electrochemical behavior of anatase TiO2 in aqueous lithium hydroxide electrolyte.
Manickam M, et al.
J. Appl. Electrochem., 36(5), 599-602 (2006)
Low-Temperature, Solution-Processed and Alkali Metal Doped ZnO for High-Performance Thin-Film Transistors.
Park SY, et al.
Advanced Mat., 24(6), 834-838 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.