추천 제품
Quality Level
분석
≥96.0% (HPLC)
양식
powder
mp
≥300 °C
>300 °C (lit.)
solubility
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
응용 분야
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
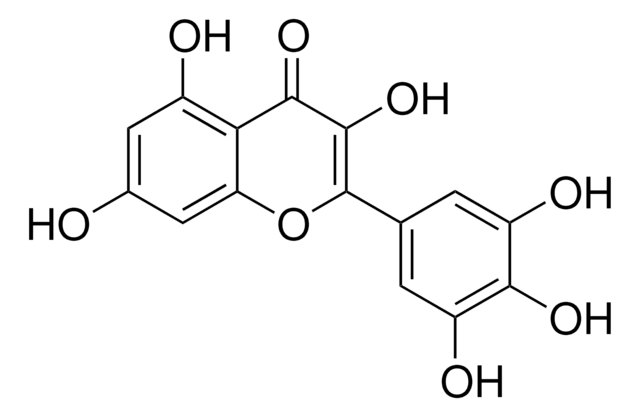
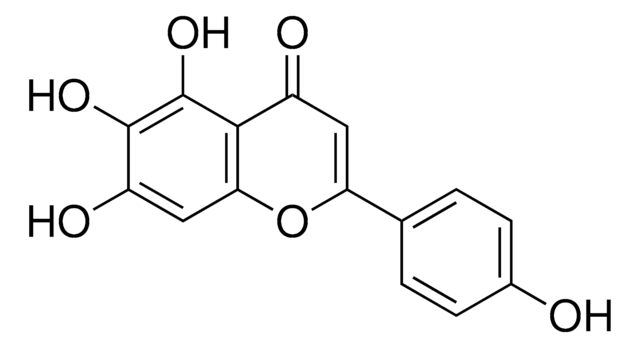
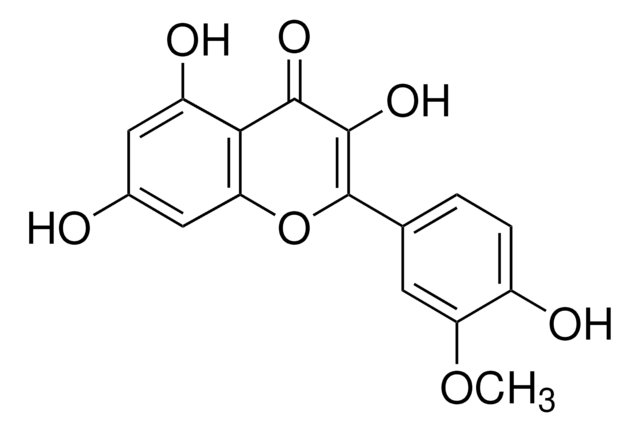
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
InChI key
IKMDFBPHZNJCSN-UHFFFAOYSA-N
유전자 정보
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
프로토콜
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.