가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
일반 설명
Penicillin amidase is a periplasmic 80K heterodimer with A and B chains (209 and 566 amino acids, respectively). It is widely distributed among microorganisms, including bacteria, yeast and filamentous fungi. Among all the sources, the enzyme produced by E. coli is most well-characterized and common for industrial application.[1]
애플리케이션
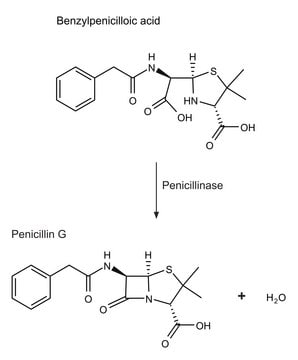
Penicillin amidase was used to study its effect in release of fatty acid and HSL (homoserine lactone) from AHLs (N -acylhomoserine lactones) in degradation of antibiotics.[2] It was used as positive control for assaying penicillin G acylase activity in the study of functional analysis of bile salt hydrolase and penicillin acylase family members in Lactobacillus sp.[3] Penicillin amidase may be used for synthesis of 6-aminopenicillanic acid from penicillin-G and for the industrial production of β-lactam antibiotics.[4]
생화학적/생리학적 작용
The biosynthesis of Penicillin amidase in E. coli by hydrophobic protein chromatography is an inducible reaction which is regulated by metabolized carbon source (e.g. polyols, carboxylic acid etc.). It is also influenced by catabolite repression.[5] It catalyzes the formation of amide bonds through an acyl-enzyme intermediate.[6]
단위 정의
1 U corresponds to the amount of enzyme which hydrolyzes 1 μmol benzylpenicillin per minute at pH 7.6 and 37°C
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Penicillin Acylase in the Industrial Production of ?-Lactam Antibiotics
Bruggink A, Roos EC, Vroom ED
Organic Process Research & Development, 2(2), 128-133 (1998)
Lavinia Dunsmore et al.
Nature chemistry, 14(7), 754-765 (2022-06-29)
Natural products that contain ortho-quinones show great potential as anticancer agents but have been largely discarded from clinical development because their redox-cycling behaviour results in general systemic toxicity. Here we report conjugation of ortho-quinones to a carrier, which simultaneously masks
A. Guy et al.
Bioorganic & Medicinal Chemistry Letters, 3, 1041-1041 (1993)
Penicillin acylase (bacterial).
T A Savidge et al.
Methods in enzymology, 43, 705-721 (1975-01-01)
Yi-Han Lin et al.
Molecular microbiology, 47(3), 849-860 (2003-01-22)
N-acylhomoserine lactones (AHLs) are used as signal molecules by many quorum-sensing Proteobacteria. Diverse plant and animal pathogens use AHLs to regulate infection and virulence functions. These signals are subject to biological inactivation by AHL-lactonases and AHL-acylases. Previously, little was known
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.