추천 제품
생화학적/생리학적 작용
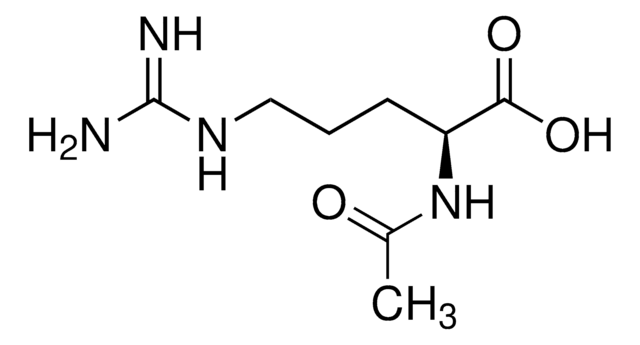
N-Acetyl-L-proline, an analog of the COOH-terminal dipeptide portion of preferred angiotensin-converting enzyme substrates, is use to probe the active site of angiotensin-converting enzyme(s). N-Acetyl-L-proline may be used to to identify, differentiate and characterized N-acyl-amino acid amidohydrolase(s)/aminoacylase(s). N-Acetyl-L-proline is used to study the physicochemical parameters of prolines.
N-acetyl-L-proline is an analog of the COOH-terminal dipeptide portion of preferred substrates of angiotensin-converting enzyme (ACE). It may be used in studies of the binding of substrates and inhibitors by ACE and to differentiate the specificities of various aminoacylases.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
J Krapcho et al.
Journal of medicinal chemistry, 31(6), 1148-1160 (1988-06-01)
Analogues of captopril, enalaprilat, and the phosphinic acid [hydroxy(4-phenylbutyl)phosphinyl]acetyl]-L-proline incorporating 4-substituted proline derivatives have been synthesized and evaluated as inhibitors of angiotensin-converting enzyme (ACE) in vitro and in vivo. The 4-substituted prolines, incorporating alkyl, aryl, alkoxy, aryloxy, alkylthio, and arylthio
Abil E Aliev et al.
The journal of physical chemistry. B, 111(50), 14034-14042 (2007-11-22)
The results of the ring conformational analysis of L-proline, N-acetyl-L-proline, and trans-4-hydroxy-L-proline by NMR combined with calculations using density functional theory (DFT) and molecular dynamics (MD) are reported. Accurate values of 1H-1H J-couplings in water and other solvents have been
Mayuko Koreishi et al.
Bioscience, biotechnology, and biochemistry, 69(10), 1914-1922 (2005-10-26)
A novel aminoacylase was purified to homogeneity from culture broth of Streptomyces mobaraensis, as evidenced by SDS-polyacrylamide gel electrophoresis (PAGE). The enzyme was a monomer with an approximate molecular mass of 100 kDa. The purified enzyme was inhibited by the
Jiyun Liu et al.
Journal of the American Chemical Society, 127(7), 2044-2045 (2005-02-17)
Structure-based design of a bifunctional ligand for two protein pentamers, cholera toxin B pentamer (CTB) and human serum amyloid P component (SAP), leads to multivalent dimerization of CTB and SAP in solution. This multivalent heterodimerization of proteins significantly enhances the
Jeanette F Kheir et al.
The journal of physical chemistry. B, 115(49), 14846-14851 (2011-11-03)
In this study, the reactions of electrons with N-acetylproline are investigated by electron spin resonance (ESR) spectroscopy and density functional theory. Electrons are produced by γ irradiation or by photoionization of K(4)Fe(CN)(6) in neutral 7.5 M LiCl-D(2)O aqueous glasses at
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.