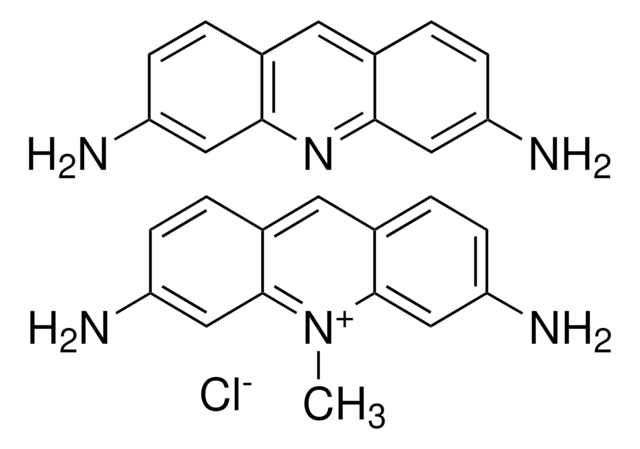
A8126
Acriflavine
Powder
동의어(들):
3,6-Diamino-10-methylacridinium chloride mixt. with 3,6-diaminoacridine (proflavine), Euflavine, Trypaflavine Neutral
About This Item
추천 제품
제품명
Acriflavine, fluorescent label
Quality Level
양식
powder
기술
microbe id | staining: suitable
mp
179-181 °C
solubility
H2O: 0.33 g/mL (lit.)(lit.)
εmax
≥48000 at 459-465 nm in methanol at 0.004 g/L
≥50000 at 259-265 nm in methanol at 0.004 g/L
응용 분야
diagnostic assay manufacturing
hematology
histology
저장 온도
room temp
SMILES string
[Cl-].Nc1ccc2cc3ccc(N)cc3nc2c1.C[n+]4c5cc(N)ccc5cc6ccc(N)cc46
InChI
1S/C14H13N3.C13H11N3.ClH/c1-17-13-7-11(15)4-2-9(13)6-10-3-5-12(16)8-14(10)17;14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10;/h2-8H,1H3,(H3,15,16);1-7H,14-15H2;1H
InChI key
PEJLNXHANOHNSU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Excitation and emission wavelengths in various solvents :
- Methanol: λex = 424 nm; λem = 518 nm
- Ethanol: λex = 426 nm; λem = 524 nm
- Propanol: λex = 430 nm; λem = 512 nm
- Butanol: λex = 430 nm; λem = 526 nm
- Formamide: λex = 434 nm; λem = 524 nm
- Glycerol: λex = 432 nm; λem = 540 nm
- Water: λex = 416 nm; λem = 514 nm
Insoluble in ether, chloroform, and fixed oils. Utilized in fluorescence steady state measurements as a donor molecule (when paired with rhodamine 6G as the acceptor) to function as a pH sensor .
- Acriflavine has been used in the agglutination test to distinguish between smooth and rough colony formation of Brucella melitensis.
- It has been used as an Ago2 (argonaute 2) inhibitor.
- It has been used as an inhibitor of HIF-ARNT (hypoxia-inducible factor - aryl hydrocarbon receptor nuclear translocator) complex formation.
- It has been used to study the bacteriocin production by Carnobacterium piscicola.
생화학적/생리학적 작용
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.