추천 제품
생물학적 소스
fungus
분석
≥98.5% (GC)
형태
liquid
작용기
ester
지질 유형
omega FAs
배송 상태
ambient
저장 온도
−20°C
SMILES string
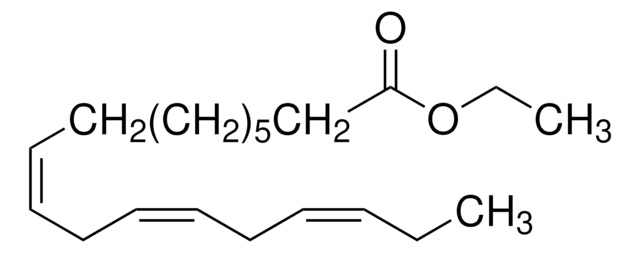
CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OCC
InChI
1S/C22H36O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24-4-2/h8-9,11-12,14-15,17-18H,3-7,10,13,16,19-21H2,1-2H3/b9-8-,12-11-,15-14-,18-17-
InChI key
SNXPWYFWAZVIAU-GKFVBPDJSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
Arachidonic acid (AA) is an unsaturated omega-6 fatty acid constituent of the phospholipids of cell membranes. Phospholipase A2 releases AA from the membrane phospholipids in response to inflammation. AA is subsequently metabolized to prostaglandins and thromboxanes by at least two cyclooxygenase (COX) isoforms, to leukotrienes and lipoxins by lipoxygenases, and to epoxyeicosatrienoic acids via p450-catalyzed metabolism. AA and its metabolites play important roles in a variety of biological processes, including signal transduction, contraction, chemotaxis, and cell proliferation, differentiation and apoptosis. AA has been demonstrated to bind to the α subunit of G protein and inhibit the activity of Ras GTPase-activating proteins (GAPs). Cellular uptake of AA is energy dependent and involves protein-facilitated transport across the plasma membrane.
포장
Sealed ampule.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Eiichiro Ishido et al.
Bioscience, biotechnology, and biochemistry, 66(1), 73-77 (2002-02-28)
Two polyunsaturated fatty acids (PUFAs) or their esters were mixed, and their oxidation processes were measured at 65 degrees C and ca. 0% relative humidity. Except when a PUFA ester was mixed with a free PUFA, the oxidation of the
D L Luthria et al.
Lipids, 28(9), 853-856 (1993-09-01)
Ethyl 5,8,11,14-eicosatetraenoate-19,19,20,20-d4 and ethyl 8,11,14-eicosatrienoate-19,19,20,20-d4 were synthesized by Grignard coupling of the methanesulfonyl ester of 2,5-undecadiyn-1-ol-10,10,11,11-d4 with 5,8-nonadiynoic acid and 8-nonynoic acid, respectively. The coupled products upon Lindlar reduction, followed by the preparation of their ethyl esters, yielded deuteriated ethyl
O Holian et al.
Biochemical and biophysical research communications, 160(3), 1110-1116 (1989-05-15)
Purified rat pancreas protein kinase C (PKC) is activated by unsaturated free fatty acids (oleic and arachidonic). The ethyl esters of these fatty acids are ineffective as enzyme activators. However, when the ethyl esters are added in combination with a
The improvement in endotoxin-induced redistribution of organ blood flow by inhibition of thromboxane and prostaglandin synthesis.
G E Tempel et al.
Advances in shock research, 7, 209-218 (1982-01-01)
Kwang-Geun Lee et al.
Journal of agricultural and food chemistry, 51(24), 7203-7207 (2003-11-13)
The antioxidant activities of naturally occurring plant compounds were measured in a lipid peroxidation system consisting of ethyl arachidonate and Fenton's reagent. Inhibitory effects of 24 plant-derived flavonoids and 5 phenolic acids on malonaldehyde (MA) formation from ethyl arachidonate were
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.