C3784
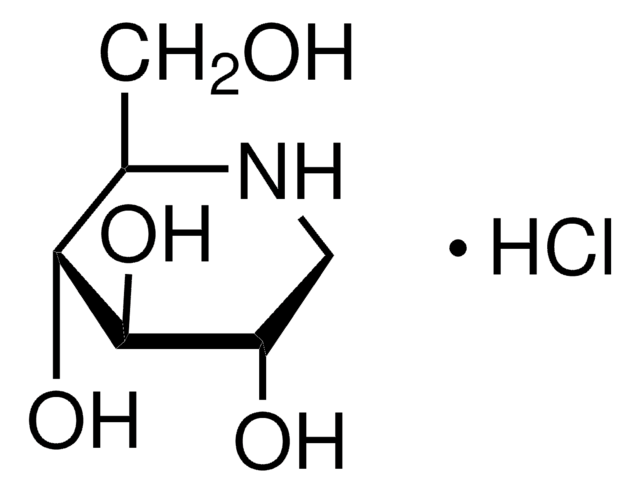
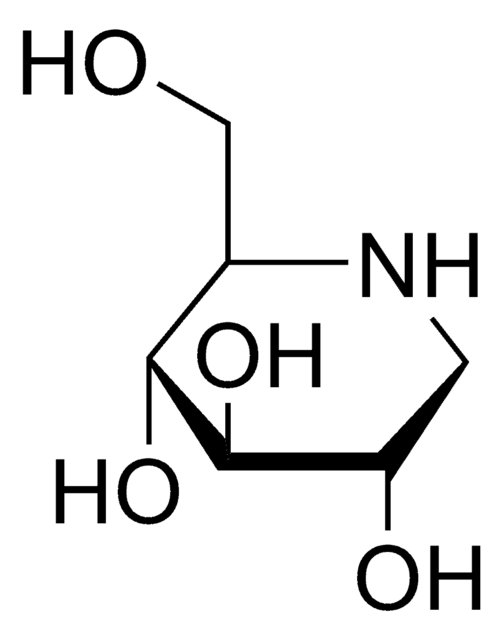
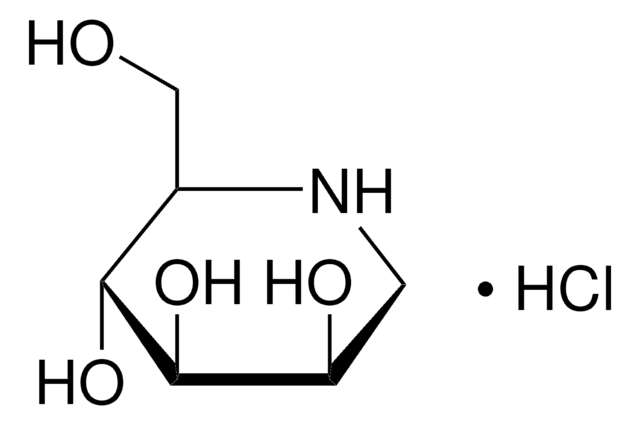
Castanospermine
≥94% (GC), BioUltra, from Castanospermum australe seeds
동의어(들):
(1S,6S,7R,8R,8aR)-1,6,7,8-Tetrahydroxyoctahydroindolizidine, (1S,6S,7R,8R,8aR)-Octahydro-1,6,7,8-indolizinetetrol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
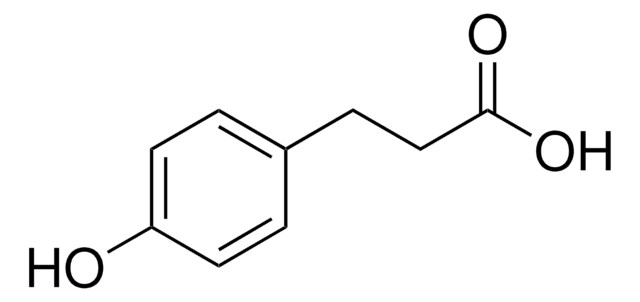
C8H15NO4
CAS Number:
Molecular Weight:
189.21
Beilstein:
3588654
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
추천 제품
생물학적 소스
Castanospermum australe seeds
제품 라인
BioUltra
분석
≥94% (GC)
양식
powder
mp
212-215 °C (dec.)
solubility
1 M HCl: 20 mg/mL, clear, colorless to faintly yellow
저장 온도
2-8°C
SMILES string
O[C@H]1CCN2C[C@H](O)[C@@H](O)[C@H](O)[C@@H]12
InChI
1S/C8H15NO4/c10-4-1-2-9-3-5(11)7(12)8(13)6(4)9/h4-8,10-13H,1-3H2/t4-,5-,6+,7+,8+/m0/s1
InChI key
JDVVGAQPNNXQDW-TVNFTVLESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
α-glucosidase Inhibitor
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
M R Bartlett et al.
Immunology and cell biology, 72(5), 367-374 (1994-10-01)
The glycoprotein processing inhibitor castanospermine (CS) and the monosaccharide mannose-6-phosphate (M6P), as well as some sulfated polysaccharides (SPS), have been shown to inhibit inflammation in rat models of experimental autoimmune encephalomyelitis and adjuvant-induced arthritis. Here, the anti-inflammatory effects of these
Tomohisa Kato et al.
Analytical biochemistry, 405(1), 103-108 (2010-06-24)
Saccharide primers, such as dodecyl beta-lactoside (Lac-C12), are unique artificial precursors of glycolipid synthesis. In culture media supplemented with saccharide primers, they are taken up by the cells in the culture media and glycosylated by cellular glycosyltransferases in the Golgi
Julien Ceccon et al.
Organic & biomolecular chemistry, 7(10), 2029-2031 (2009-05-08)
An asymmetric synthesis of (+)-castanospermine is presented in which enol ether metathesis-hydroboration/oxidation is used for stereoselective installation of the trans-trans hydroxyl groups on the piperidine ring of the alkaloid.
Matilde Aguilar-Moncayo et al.
Organic & biomolecular chemistry, 7(13), 2738-2747 (2009-06-18)
Synthesis of a panel of iso(thio)urea-type ring-modified castanospermine analogues bearing a freely mutarotating pseudoanomeric hydroxyl group results in tight-binding beta-glucosidase inhibitors with unusual binding signatures; the presence of an N-octyl substituent imparts a remarkable anomeric selectivity, promoting strong binding of
Thomas Jensen et al.
The Journal of organic chemistry, 74(22), 8886-8889 (2009-10-28)
A nine-step synthesis of (+)-castanospermine has been accomplished in 22% overall yield from methyl alpha-D-glucopyranoside. The key transformations involve a zinc-mediated fragmentation of benzyl-protected methyl 6-iodoglucopyranoside, ring-closing olefin metathesis, and strain-release transannular cyclization to afford the indolizidine skeleton of the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.