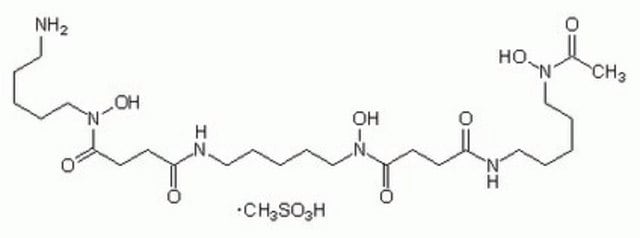
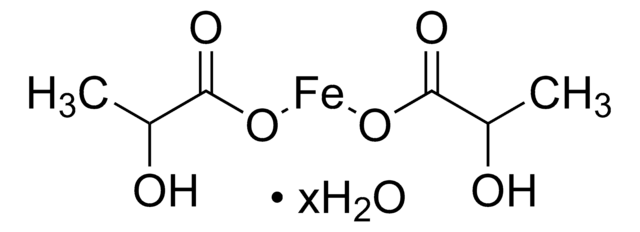
추천 제품
애플리케이션
Iron-Dextran (ferric hydroxide dextran complex) may be used as an intravenous iron delivery preparation. Iron-Dextran may be used to induce iron-overload to study its effects and preventative measures.
기타 정보
To gain a comprehensive understanding of our extensive range of Dextrans for your research, we encourage you to visit our Carbohydrates Category page.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 1B - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Zachariah DeFilipp et al.
Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery, 9(1), 129-132 (2012-08-08)
Iron deficiency is a major postoperative complication of Roux-en-Y gastric bypass surgery. Oral replacement can fail to correct the deficiency. Thus, recourse to parenteral iron administration might be necessary. Our objective was to evaluate the effectiveness and safety of a
Maureen M Okam et al.
American journal of hematology, 87(11), E123-E124 (2012-09-12)
Oral iron replacement is the standard therapy in iron-deficiency anemia (IDA). However, 59% of patients have gastrointestinal toxicity. With impaired iron uptake from the gastrointestinal tract (in anemia of chronic disease (ACD) or after bariatric surgery), suboptimal responsiveness to exogenous
C Egger et al.
Ultraschall in der Medizin (Stuttgart, Germany : 1980), 33(6), 587-592 (2012-11-17)
To check the feasibility of the easy quantification of tumor vascularization derived from dynamic contrast-enhanced ultrasound (DCE-US) in comparison to dynamic contrast-enhanced computed tomography (DCE-CT) in patients with hepatocellular carcinoma (HCC). 19 patients with cirrhosis and histologically proven HCC prospectively
V J Kumpf et al.
DICP : the annals of pharmacotherapy, 24(2), 162-166 (1990-02-01)
Parenteral iron therapy is indicated in patients with iron-deficiency anemia associated with conditions that interfere with the ingestion or absorption of oral iron. Replacement doses of iron required to replenish iron stores are based on body weight and the observed
Markus R Jahn et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 78(3), 480-491 (2011-03-29)
The treatment of iron deficiency anemia with polynuclear iron formulations is an established therapy in patients with chronic kidney disease but also in other disease areas like gastroenterology, cardiology, oncology, pre/post operatively and obstetrics' and gynecology. Parenteral iron formulations represent
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.