추천 제품
애플리케이션
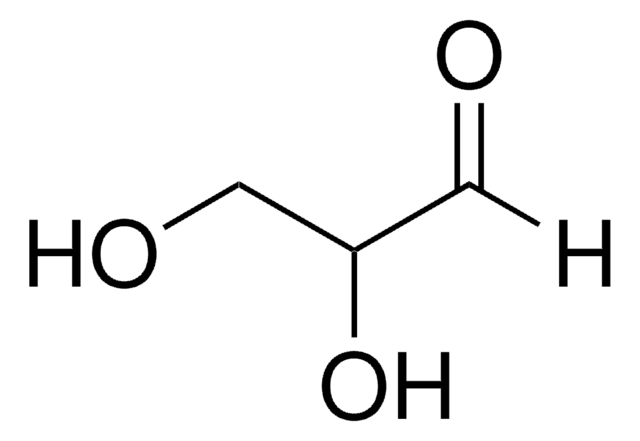
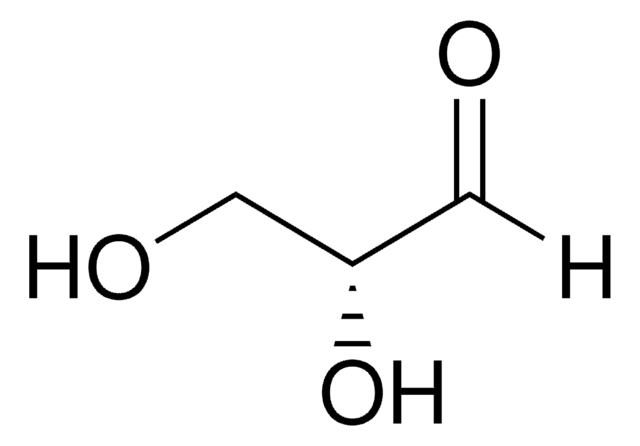
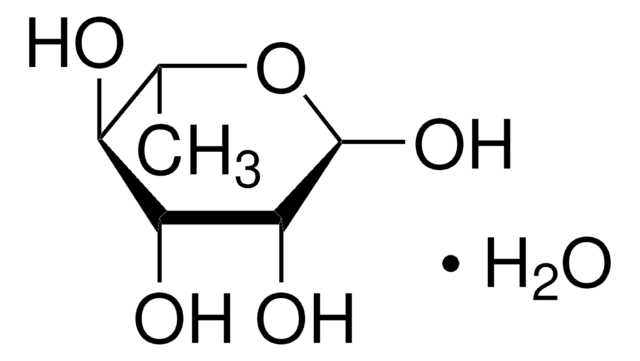
D-(-)-Erythrose, the D enantiomer of the aldose aldehyde erythrose, may be used as a reference compound in sugar metabolism analysis. D-(-)-Erythrose may be used to help identify and characterize erythrose reductase(s). D-Erythrose may be used to study the mechanisms of mutarotation in monosugars. D-Erythrose may be used to study the mechanisms of organic microspherule formation and Maillard (glycation) reactions.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Dae-Hee Lee et al.
Microbial cell factories, 9, 43-43 (2010-06-10)
Erythrose reductase (ER) catalyzes the final step of erythritol production, which is reducing erythrose to erythritol using NAD(P)H as a cofactor. ER has gained interest because of its importance in the production of erythritol, which has extremely low digestibility and
Arthur L Weber
Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life, 35(6), 523-536 (2005-10-29)
Reaction of small sugars of less than four carbons with ammonia in water yielded organic microspherules generally less than ten microns in size. The time course of microspherule growth was examined for the D-erythrose-ammonia reaction that yielded microspherules attached to
Julia Schörghuber et al.
Journal of biomolecular NMR, 71(3), 129-140 (2018-05-29)
In recent years, we developed a toolbox of heavy isotope containing compounds, which serve as metabolic amino acid precursors in the E. coli-based overexpression of aromatic residue labeled proteins. Our labeling techniques show excellent results both in terms of selectivity
Oliver Reihl et al.
Carbohydrate research, 339(9), 1609-1618 (2004-06-09)
Covalently cross-linked proteins are among the major modifications caused by the advanced Maillard reaction. In the present study, the formation pathway of the dideoxyosone N6-(2,3-dihydroxy-5,6-dioxohexyl)-L-lysine is shown. To elucidate the formation of this glucose-derived dideoxyosone D-lactose (O-beta-D-galp-(1-->4)-D-glcp) and D-glucose-6-phosphate were
Chaoyi Chang et al.
The Journal of chemical physics, 153(4), 044126-044126 (2020-08-06)
Elementary steps and intermediate species of linearly structured biomass compounds are studied. Specifically, possible intermediates and elementary reactions of 15 key biomass compounds and 33 small molecules are obtained from a recursive bond-breaking algorithm. These are used as inputs to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.