추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
solubility
DMSO: >20 mg/mL
주관자
Novartis
저장 온도
room temp
SMILES string
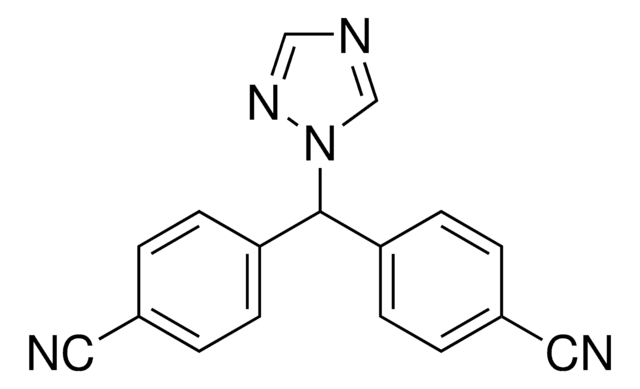
Cl.N#Cc1ccc(cc1)C2CCCc3cncn23
InChI
1S/C14H13N3.ClH/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14;/h4-7,9-10,14H,1-3H2;1H
InChI key
UKCVAQGKEOJTSR-UHFFFAOYSA-N
생화학적/생리학적 작용
Fadrozole is a nonsteroidal aromatase inhibitor.
Fadrozole is a nonsteroidal aromatase inhibitor. Fadrozole is a very potent and highly selective inhibitor of the aromatase enzyme system in vitro and estrogen biosynthesis in vivo. It inhibited the conversion of [4-14C]androstenedione to [4-14C]estrone by human placental microsomes in a competitive manner (Ki = 1.6 nM). At a substrate concentration 3-fold the Km, Fadrozole was 180 times more potent, as an inhibitor, than aminoglutethimide (Cat. No. A9657), exhibiting half-maximal inhibition at 1.7 nM as compared to 0.3 μM. In vivo, Fadrozole lowered ovarian estrogen synthesis by gonadotropin-primed, androstenedione treated, immature rats by 90% at a dose of 260 μg/kg (PO). In vivo, Fadrozole leads to sequelae of estrogen deprivation (e.g. regression of DMBA-induced mammary tumors) without causing adrenal hypertrophy in adult rats. It blocked aromatase by 50% in human breast cancer homogenates, live breast cancer cells, human placental microsomes, and porcine ovarian microsomes at concentrations of 0.008 to 0.02 μM.
특징 및 장점
This compound was developed by Novartis. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Thierry D Charlier et al.
General and comparative endocrinology, 167(1), 18-26 (2010-02-11)
Local aromatization of testosterone into 17beta-estradiol (E(2)) is often required for the physiological and behavioral actions of testosterone. In most vertebrates, aromatase is expressed in a few discrete brain regions. While many studies have measured brain aromatase mRNA or activity
Devy Deliyanti et al.
Hypertension (Dallas, Tex. : 1979), 59(3), 607-613 (2012-01-26)
Neovascularization is a hallmark feature of retinopathy of prematurity and diabetic retinopathy. Type 1 angiotensin receptor blockade reduces neovascularization in experimental retinopathy of prematurity, known as oxygen-induced retinopathy (OIR). We investigated in OIR whether inhibiting aldosterone with the aldosterone synthase
Elizabeth Adkins-Regan
Frontiers in neuroendocrinology, 32(2), 155-163 (2011-02-01)
A majority of birds are socially monogamous, providing exceptional opportunities to discover neuroendocrine mechanisms underlying preferences for opposite-sex partners where the sexes form extended affiliative relationships. Zebra finches have been the focus of the most systematic program of research to
Aviva Gamliel-Lazarovich et al.
Journal of hypertension, 28(9), 1900-1907 (2010-08-12)
Aldosterone is known to be involved in atherosclerosis and cardiovascular disease and blockade of its receptor was shown to improve cardiovascular function. It was, therefore, hypothesized that inhibition of aldosterone synthesis would also reduce atherosclerosis development. To test this hypothesis
Luke Remage-Healey et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(24), 8231-8241 (2012-06-16)
The activity of sensory circuits is shaped by neuromodulators, which can have downstream consequences for both sensorimotor integration and behavioral output. Recent evidence indicates that brain-derived estrogens ("neuroestrogens") can act as local circuit modulators in the songbird auditory forebrain. Specifically
문서
We offers many products related to Nuclear Receptors (Steroids) for your research needs.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.