추천 제품
생물학적 소스
bacterial (Proteus spp.)
Quality Level
양식
buffered aqueous solution
특이 활성도
≥4,000 units/mL
분자량
~300 kDa
저장 온도
2-8°C
애플리케이션
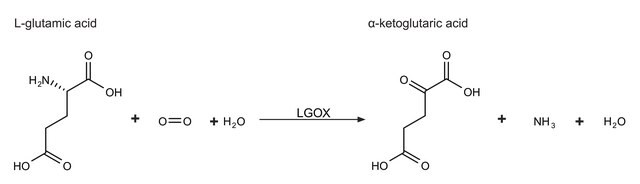
This enzyme is useful for enzymatic determination of NH3, α-ketoglutaric acid and L-glutamic acid, and for assay of leucine aminopeptidase and urease. This enzyme is also used for enzymatic determination of urea when coupled with urease (URH-201) in clinical analysis. In vitro, various activity assays of this enzyme examine the conversion of α-ketoglutarate to L-glutamate, in the presence of excess ammonium ions (NH4+) and NADPH.
생화학적/생리학적 작용
L-glutamic dehydrogenase catalyzes the conversion of glutamate to α-ketoglutarate.
물리적 특성
Isoelectric point : 4.6
Michaelis constants : 1.1 X 10-3M (NH3), 3.4 X 10-4M (α-Ketoglutarate)
1.2 X 10-3M (L-Glutamate), 1.4 X 10-5M (NADPH), 1.5 X 10-5M (NADP+)
Structure : 6 subunits (M.W.50,000) per mol of enzyme
Inhibitors : Hg++, Cd++, p-chloromercuribenzoate, pyridine, 4-4′-dithiopyridine,
2,2′-dithiopyridine
Optimum pH : 8.5 (α-KG→L-Glu) 9.8 (L-Glu→α-KG)
Optimum temperature : 45oC(α-KG−L-Glu) 45-55oC (L-Glu→α-KG)
pH stability : pH 6.0 - 8.5 (25oC, 20hr)
Thermal stability : below 50oC (pH 7.4, 10min)
Michaelis constants : 1.1 X 10-3M (NH3), 3.4 X 10-4M (α-Ketoglutarate)
1.2 X 10-3M (L-Glutamate), 1.4 X 10-5M (NADPH), 1.5 X 10-5M (NADP+)
Structure : 6 subunits (M.W.50,000) per mol of enzyme
Inhibitors : Hg++, Cd++, p-chloromercuribenzoate, pyridine, 4-4′-dithiopyridine,
2,2′-dithiopyridine
Optimum pH : 8.5 (α-KG→L-Glu) 9.8 (L-Glu→α-KG)
Optimum temperature : 45oC(α-KG−L-Glu) 45-55oC (L-Glu→α-KG)
pH stability : pH 6.0 - 8.5 (25oC, 20hr)
Thermal stability : below 50oC (pH 7.4, 10min)
단위 정의
One unit will reduce 1.0 μmole of α-ketoglutarate to L-glutamate per min at pH 8.3 at 30 °C in the presence of ammonium ions and NADPH.
물리적 형태
Solution in 50 mM Tris HCl, pH 7.8, 5 mM Na2EDTA containing 0.05% sodium azide
기타 정보
Note: Do not confuse with non-specific L-GLDH, EC 1.4.1.3.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
D P Hornby et al.
The Biochemical journal, 223(1), 161-168 (1984-10-01)
In steady-state kinetic studies of ox liver glutamate dehydrogenase in 0.11 M-potassium phosphate buffer, pH7, at 25 degrees C, the concentration of ADP was varied from 0.5 to 1000 microM. Inhibition was observed except when the concentrations of both glutamate
J Bailey et al.
The Journal of biological chemistry, 257(10), 5579-5583 (1982-05-25)
The activity of bovine liver glutamate dehydrogenase is affected in several ways depending on substrate concentrations and pH. At ph 6.5 and below, both oxidative deamination and reductive amination reactions are inhibited by ADP. At pH 7.0 and above both
Daria V Borsakova et al.
Scientific reports, 12(1), 5437-5437 (2022-04-02)
Excessive ammonium blood concentration causes many serious neurological complications. The medications currently used are not very effective. To remove ammonium from the blood, erythrocyte-bioreactors containing enzymes that processing ammonium have been proposed. The most promising bioreactor contained co-encapsulated glutamate dehydrogenase
Wei-Li Liao et al.
Fungal genetics and biology : FG & B, 45(4), 514-526 (2007-10-24)
The function of GLN3, a GATA factor encoding gene, in nitrogen metabolism of Candida albicans was examined. GLN3 null mutants had reduced growth rates on multiple nitrogen sources. More severe growth defects were observed in mutants lacking both GLN3 and
S Cossy Isasi et al.
Experimental parasitology, 122(3), 218-225 (2009-04-09)
Biochemical and structural modifications were investigated in axenic cultured Trypanosoma cruzi after treatment with gangliosides. Fluorescence anisotropy showed dose dependent increments in parasite membranes of ganglioside treated epimastigotes. NADP-GDH activity increased in parasites treated at day 4 (13%), 7 (137.2%)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.