K0133
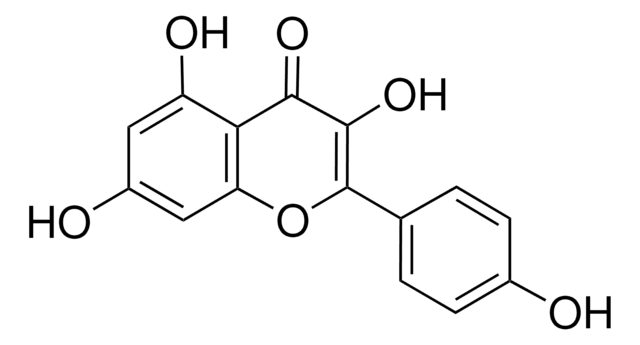
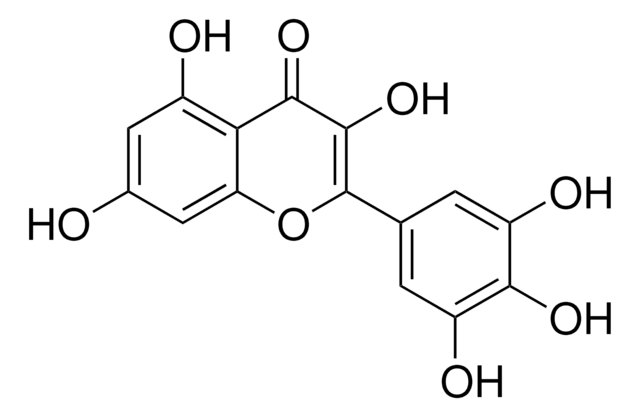
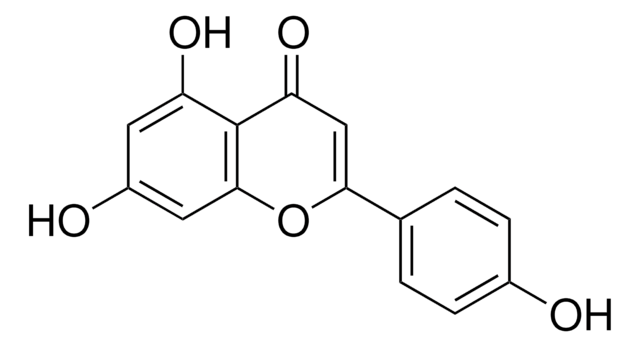
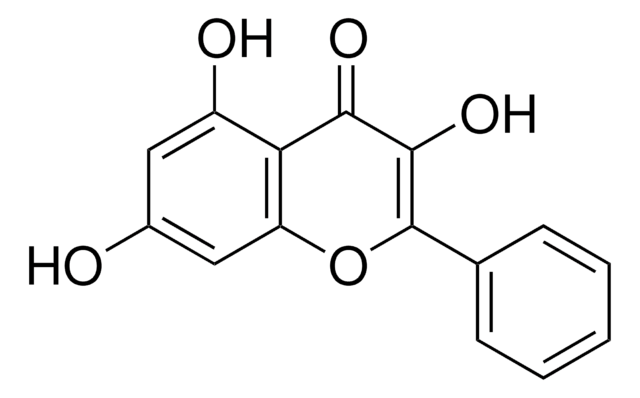
Kaempferol
≥90% (HPLC), powder
동의어(들):
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
추천 제품
생물학적 소스
synthetic
Quality Level
분석
≥90% (HPLC)
형태
powder
저장 조건
protect from light
색상
yellow
mp
277 °C
solubility
ethanol: 20 mg/mL
DMSO: 50 mg/mL
저장 온도
room temp
SMILES string
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
InChI key
IYRMWMYZSQPJKC-UHFFFAOYSA-N
유전자 정보
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- to check its potential effect as an antioxidant and neuroprotective agent against rotenone-induced Parkinson′s disease (PD) model in SH-S5Y5 cells
- to test its anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammatory injury in human aortic endothelial cells (HAECs)
- to study its apoptosis sensitizing effect on non-small cell lung cancer (NSCLC) cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2)
생화학적/생리학적 작용
포장
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.