S0625
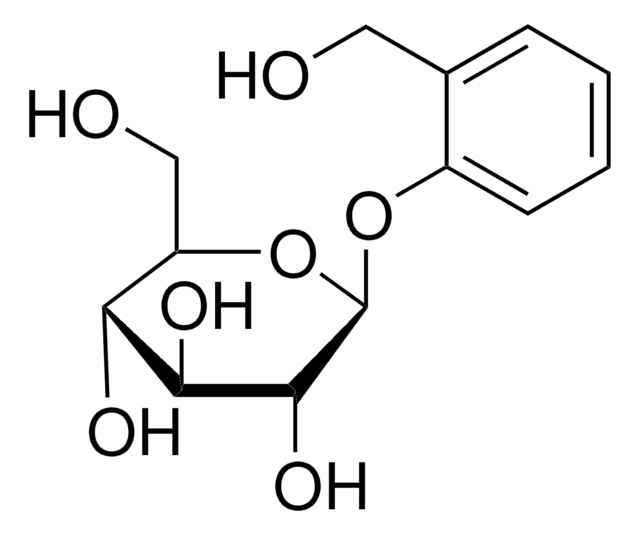
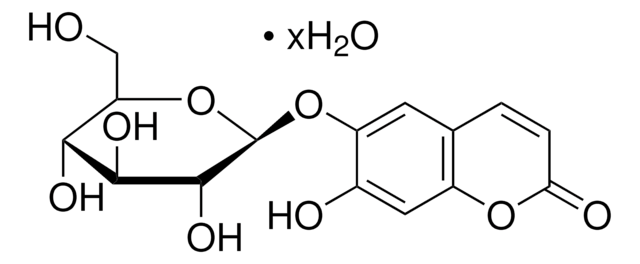
D-(−)-Salicin
≥99% (GC)
동의어(들):
2-(Hydroxymethyl)phenyl-β-D-glucopyranoside, Salicoside, Salicyl alcohol glucoside, Saligenin β-D-glucoside
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
실험식(Hill 표기법):
C13H18O7
CAS Number:
Molecular Weight:
286.28
Beilstein:
89593
EC Number:
MDL number:
UNSPSC 코드:
12352205
PubChem Substance ID:
NACRES:
NA.25
추천 제품
Quality Level
분석
≥99% (GC)
양식
powder
응용 분야
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
OC[C@H]1O[C@@H](Oc2ccccc2CO)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C13H18O7/c14-5-7-3-1-2-4-8(7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-4,9-18H,5-6H2/t9-,10-,11+,12-,13-/m1/s1
InChI key
NGFMICBWJRZIBI-UJPOAAIJSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Salicin is a non-phenolic glucosidic compound extracted from meadowsweet (Filipendula ulmaria L). It is majorly used as a substitute for quinine. Salicin can be used as a therapeutic for patients suffering from rheumatic fever. Salicin acts a metabolic precursor for salicylic acid. It is a novel phytochemical that exhibits immunological cross functions in plants and humans. Salicin facilitates growth and reproduction in plants. In addition, It also protects plants against biotic and abiotic stress.
애플리케이션
D-(-)-Salicin has been used:
- to study its in vitro anticoagulant and antiplatelet activities
- as a standard in high performance liquid chromatography method (HPLC) for quantitation of salicin from willow plant
- as a tastant in taste threshold assay
- as a constituent of nutrient agar-salicin medium for selective isolation of Lactobacillus paracasei
생화학적/생리학적 작용
D-(−)-Salicin exerts anti-rheumatism and anti-inflammatory activities. It inhibits lipopolysaccharide (LPS) induced inflammation in in vitro and in vivo studies. It regulates different signaling pathways such as nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK). It also regulates cytokines concentration such as tumor necrosis factor-alpha(TNF-α), interleukin-1β, interleukin-6, and interleukin-10.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Autofluorescence-based identification and functional validation of antennal gustatory sensilla in a specialist leaf beetle
Pentzold S, et al.
Frontiers in Physiology, 10, 343-343 (2019)
Evaluation of culture media for selective enumeration of probiotic strains of lactobacilli and bifidobacteria in combination with yoghurt or cheese starters
Van de Casteele S, et al.
International dairy journal, 16(12), 1470-1476 (2006)
Maik Behrens et al.
Chemical senses, 38(6), 475-484 (2013-05-02)
Mutational polymorphism in the TAS2R38 bitter taste receptor is a key determinant of threshold taste detection of isolated compounds, such as phenylthiocarbamide (PTC) and propylthiouracil (PROP), as well as complex orosensation-mediated traits such as diet choice and smoking habits. These
Genetics and bitter taste responses to goitrin, a plant toxin found in vegetables
Wooding S, et al.
Chemical Senses, 35(8), 685-692 (2010)
Drug discovery: a history (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.