모든 사진(1)
About This Item
실험식(Hill 표기법):
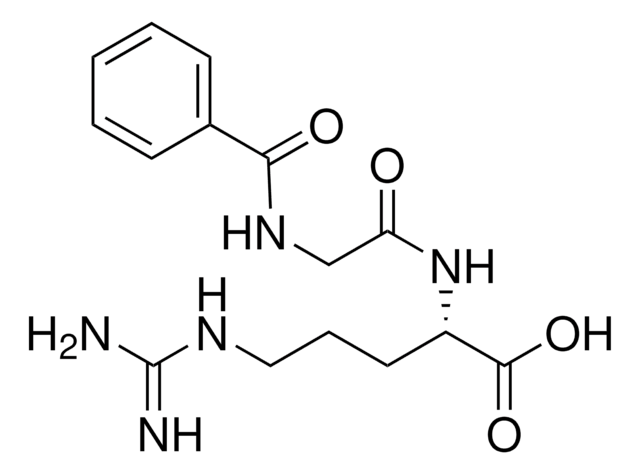
C40H53N5O10
CAS Number:
Molecular Weight:
763.88
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥90% (HPLC)
양식
powder
solubility
0.1% trifluoroacetic acid in acetonitrile: water (3:1): 1 mg/mL, clear, colorless
저장 온도
−20°C
SMILES string
CC(C)CC(NC(=O)CCC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(Cc1ccc(O)cc1)C(=O)Nc2ccc3C(C)=CC(=O)Oc3c2
InChI
1S/C40H53N5O10/c1-21(2)16-29(42-33(47)14-15-34(48)49)38(52)43-30(17-22(3)4)39(53)45-36(23(5)6)40(54)44-31(19-25-8-11-27(46)12-9-25)37(51)41-26-10-13-28-24(7)18-35(50)55-32(28)20-26/h8-13,18,20-23,29-31,36,46H,14-17,19H2,1-7H3,(H,41,51)(H,42,47)(H,43,52)(H,44,54)(H,45,53)(H,48,49)
InChI key
UVFAEQZFLBGVRM-UHFFFAOYSA-N
Amino Acid Sequence
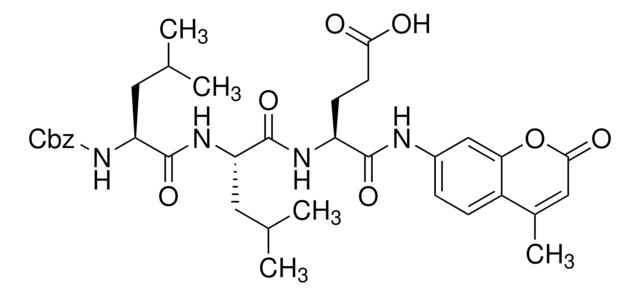
N-Suc-Leu-Leu-Val-Tyr-7-AMC
애플리케이션
N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin was used in proteasome chymotrypsin-like activity assay in Jurkat cell lysate6 and crude cell lysate of rice.7
생화학적/생리학적 작용
In the presence of chymotrypsin-like enzyme activity, the fluorophore, 7-amido-4-methylcoumarin is released from N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin. The fluorescence obtained is a measure of the enzyme activity.6
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기질
Fluorogenic substrate for chymotrypsin-like enzymes, such as cathepsin B and calpain which have been implicated in programmed cell death.
이미 열람한 고객
O Ullrich et al.
Proceedings of the National Academy of Sciences of the United States of America, 96(11), 6223-6228 (1999-05-26)
The 20S proteasome has been shown to be largely responsible for the degradation of oxidatively modified proteins in the cytoplasm. Nuclear proteins are also subject to oxidation, and the nucleus of mammalian cells contains proteasome. In human beings, tumor cells
C L Edelstein et al.
Kidney international, 50(4), 1150-1157 (1996-10-01)
The effect of the newly developed, nonpeptide, calpain inhibitor, PD 150606, on hypoxia and ionomycin-induced increases in calpain activity in rat proximal tubules (PT) was determined. PD150606 inhibited both hypoxia and ionomycin-induced calpain activity as determined by the fluorescent substrate
M Kroll et al.
Chemistry & biology, 6(10), 689-698 (1999-10-06)
The fungal epipolythiodioxopiperazine metabolite gliotoxin has a variety of toxic effects such as suppression of antigen processing, induction of macrophagocytic apoptosis and inhibition of transcription factor NF-kappaB activation. How gliotoxin acts remains poorly understood except that the molecule's characteristic disulfide
D E Atsma et al.
Circulation research, 76(6), 1071-1078 (1995-06-01)
Calcium-activated neutral protease (CANP), also known as calpain, has been implicated in the development of cell death in ischemic hearts. CANP is thought to be activated by the calcium overload that develops during ischemia. We studied the involvement of CANP
Turnover of oxidatively damaged nuclear proteins in BV-2 microglial cells is linked to their activation state by poly-ADP-ribose polymerase.
O Ullrich et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 15(8), 1460-1462 (2001-06-02)
문서
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
관련 콘텐츠
일산화질소 (NO) 및 세포 스트레스 연구를 위한 생체 활성 저분자
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.