추천 제품
생물학적 소스
Fusarium subglutinans
Quality Level
분석
≥98% (HPLC)
형태
powder
solubility
DMSO: 0.5 mg/mL (may require sonication and heating)
chloroform: 0.5 mg/mL
dichloromethane: 0.5 mg/mL
저장 온도
−20°C
InChI
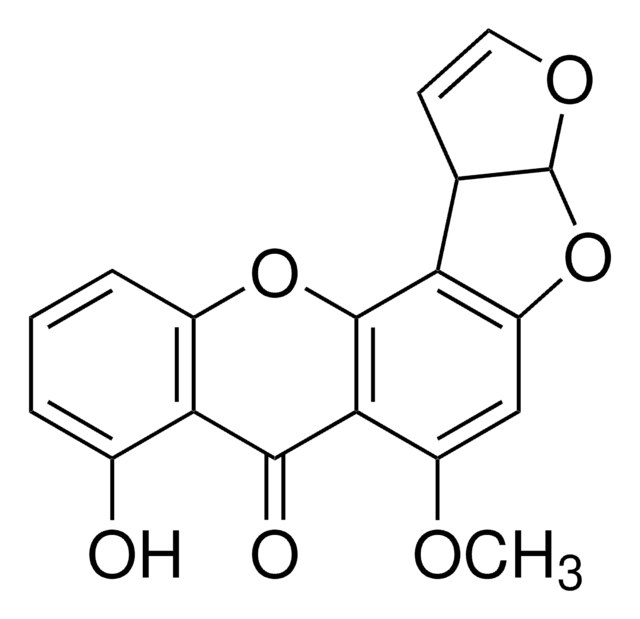
1S/C20H14O8/c1-7-4-8(26-2)5-10-12(7)17(23)15-18(24)13-9(21)6-11(27-3)16(22)14(13)19(25)20(15)28-10/h4-6,24-25H,1-3H3
InChI key
ZOQMSOSJEWBMHP-UHFFFAOYSA-N
생화학적/생리학적 작용
Bikaverin is a red pigment with a polyketide tetracyclic benzoxanthone structure. Bikaverin has an antibiotic activity against some protozoa and fungi and also inhibits Succinate- and NAD-linked respiration in rat mitochondria at 20 mg/mL. At higher concentrations (50 mg/mL) it acts as an oxidative phosphorylation uncoupling agent of tumor cells and isolated rat liver mitochondria. Bikaverin demonstrates antitumor activity on Erlich ascites carcinoma (EAC), leukemia and sarcoma cells.
재구성
Soluble in DMSO (0.5 mg/mL, may require sonication and heating). For further dilution, water based solutions can be used. Chloroform (0.5 mg/mL) and dichloromethane (0.5 mg/mL).
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Alejandro F Estrada et al.
Fungal genetics and biology : FG & B, 45(5), 705-718 (2008-01-22)
The fungal proteins of the White Collar photoreceptor family, represented by WC-1 from Neurospora crassa, mediate the control by light of different biochemical and developmental processes, such as carotenogenesis or sporulation. Carotenoid biosynthesis is induced by light in the gibberellin-producing
A G Medentsev et al.
Prikladnaia biokhimiia i mikrobiologiia, 41(5), 573-577 (2005-10-26)
We studied biosynthesis of colored naphthoquinone metabolites by Fusarium decemcellulare, F. graminearum, and F. bulbigenum fungi. F. bulbigenum and F. graminearum synthesized bikaverin and aurofusarin, respectively, which depended on the conditions of cultivation. F. decemcellulare synthesized soluble extracellular naphthoquinones of
Roberto Rodríguez-Ortiz et al.
Applied microbiology and biotechnology, 85(6), 1991-2000 (2009-10-20)
The fungus Fusarium fujikuroi (Gibberella fujikuroi mating group C) exhibits a rich secondary metabolism that includes the synthesis of compounds of biotechnological interest, such as gibberellins, bikaverin, and carotenoids. The effect of the carbon source on their production was checked
Enzymatic synthesis of aromatic polyketides using PKS4 from Gibberella fujikuroi.
Suzanne M Ma et al.
Journal of the American Chemical Society, 129(35), 10642-10643 (2007-08-19)
Pia Linnemannstöns et al.
Fungal genetics and biology : FG & B, 37(2), 134-148 (2002-11-01)
The ascomycete Gibberella fujikuroi mating population C (MP-C) is well known for the production of gibberellins, but also produces many other secondary metabolites, including the red polyketide pigment bikaverin. Here, we used a differential display method to clone a polyketide
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.