추천 제품
Quality Level
분석
≥98% (HPLC)
형태
powder
저장 조건
desiccated
색상
white to beige
solubility
H2O: 5 mg/mL, clear (warmed)
저장 온도
2-8°C
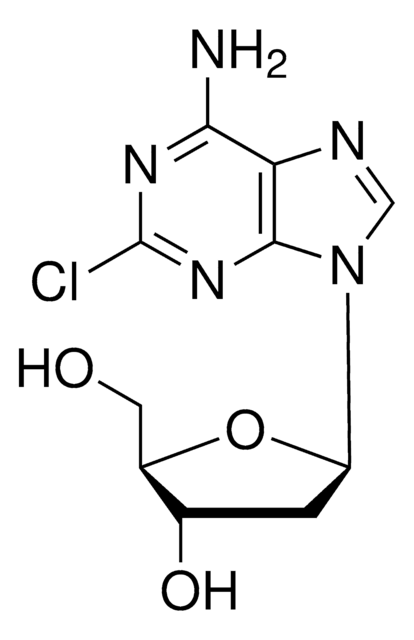
SMILES string
O[C@@H]1[C@@H](O[C@H](CO)[C@H]1O)N2C=NC3=C2N=C(N)N=C3OC
InChI
1S/C11H15N5O5/c1-20-9-5-8(14-11(12)15-9)16(3-13-5)10-7(19)6(18)4(2-17)21-10/h3-4,6-7,10,17-19H,2H2,1H3,(H2,12,14,15)/t4-,6-,7+,10?/m1/s1
InChI key
IXOXBSCIXZEQEQ-KBNQYOMWSA-N
생화학적/생리학적 작용
Nelarabine is an antineoplastic purine nucleoside analog used in T-cell acute lymphoblastic leukemia. It is a pro-drug of Ara-G, which is converted by cellular kinases to the active 5′-triphosphate, Ara-GTP. Incorporation of Ara-GTP into DNA leads to inhibition of DNA synthesis and apoptosis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Nelarabine, cyclosphosphamide and etoposide for adults with relapsed T-cell acute lymphoblastic leukaemia and lymphoma.
Marlise R Luskin et al.
British journal of haematology, 174(2), 332-334 (2015-09-26)
Stuart S Winter et al.
Pediatric blood & cancer, 62(7), 1176-1183 (2015-03-11)
Nelarabine has shown impressive single agent clinical activity in T-cell acute lymphoblastic leukemia (T-ALL), but has been associated with significant neurotoxicities in heavily pre-treated patients. We showed previously that it was safe to add nelarabine to a BFM-86 chemotherapy backbone
Larry W Buie et al.
Clinical therapeutics, 29(9), 1887-1899 (2007-11-24)
Nelarabine was approved by the US Food and Drug Administration (FDA) in October 2005 for the treatment of T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) that has not responded to or has relapsed after treatment with at
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.