SML2710
ZCZ011
≥98% (HPLC)
동의어(들):
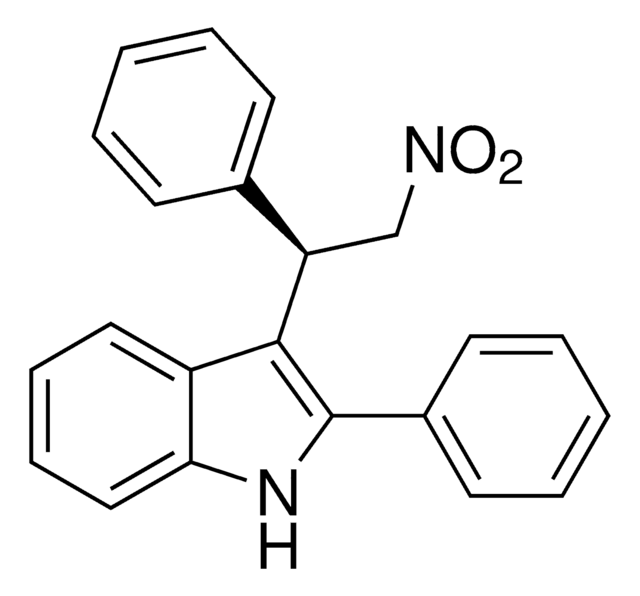
6-Methyl-3-(2-nitro-1-(thiophen-2-yl)ethyl)-2-phenyl-1H-indole, 6-Methyl-3-[2-nitro-1-(2-thienyl)ethyl]-2-phenyl-1H-indole, ZCZ 011, ZCZ-011
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H18N2O2S
CAS Number:
Molecular Weight:
362.44
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
2-8°C
SMILES string
[s]1c(ccc1)C(C[N+](=O)[O-])c2c3c([nH]c2c4ccccc4)cc(cc3)C
InChI key
RJSPNRDBWHHFMH-UHFFFAOYSA-N
생화학적/생리학적 작용
Brain-penetrant, cannabinoid receptor CB1-selective positive allosteric modulator (PAM) that blocks neuropathic pain without cannabimimetic side-effects.
ZCZ011 is a brain-penetrant, cannabinoid receptor CB1-selective positive allosteric modulator (PAM) that enhances orthosteric agonist CP55,940, while reduces orthosteric inverse agonist SR141716A, CB1 binding. ZCZ011 potentiates AEA-stimulated GTPγS binding (Emax = 46.5% without vs. 115.2% with 100 nM ZCZ011; mouse brain membranes) and cellular signaling (β-arrestin Emax = 100% without vs.157% with 100 nM ZCZ011, pERK pIC50 = 6.5 without vs. 8.3 with 10 nM ZCZ011; hCB1 cells). ZCZ011 exhibits antinociceptive efficay in murine chronic constriction injury (CCI) model of neuropathic pain & carrageenan model of inflammatory pain (40 mg/kg i.p.) without cannabimimetic side-effects.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Enantiomer-specific positive allosteric modulation of CB1 signaling in autaptic hippocampal neurons.
Jose Mitjavila et al.
Pharmacological research, 129, 475-481 (2017-11-22)
The cannabinoid signaling system is found throughout the CNS and its involvement in several pathological processes makes it an attractive therapeutic target. Because orthosteric CB1 cannabinoid receptor ligands have undesirable adverse effects there has been great interest in the development
Noureldin Saleh et al.
Angewandte Chemie (International ed. in English), 57(10), 2580-2585 (2018-01-10)
The cannabinoid CB1 receptor (CB1R) is an abundant metabotropic G-protein-coupled receptor that has been difficult to address therapeutically because of CNS side effects exerted by orthosteric drug candidates. Recent efforts have focused on developing allosteric modulators that target CB1R. Compounds
Chih-Chung Tseng et al.
Journal of medicinal chemistry, 62(10), 5049-5062 (2019-05-06)
The first generation of CB1 positive allosteric modulators (e.g., ZCZ011) featured a 3-nitroalkyl-2-phenyl-indole structure. Although a small number of drugs include the nitro group, it is generally not regarded as being "drug-like", and this is particularly true for aliphatic nitro
K R Trexler et al.
Pharmacology, biochemistry, and behavior, 177, 27-33 (2019-01-01)
Recently, multiple compounds have been synthesized that target the allosteric binding site(s) of CB1. These CB1 positive allosteric modulators may capture the benefits of cannabinoid receptor activation without unwanted psychoactive effects, such as sedation. For example, ZCZ011 blocks neuropathic pain
Kaavya Krishna Kumar et al.
Cell, 176(3), 448-458 (2019-01-15)
Cannabis elicits its mood-enhancing and analgesic effects through the cannabinoid receptor 1 (CB1), a G protein-coupled receptor (GPCR) that signals primarily through the adenylyl cyclase-inhibiting heterotrimeric G protein Gi. Activation of CB1-Gi signaling pathways holds potential for treating a number of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.