SML3300
TB21007
≥98% (HPLC)
동의어(들):
3-(2-Hydroxyethylthio)-6,6-dimethyl-1-(thiazol-2-yl)-6,7-dihydrobenzo[c]thiophen-4(5H)-one, 6,6-Dimethyl-3-(2-hydroxyethyl)thio-1-(thiazol-2-yl)-6,7-dihydro-2-benzothiophen-4(5H)-one, 6,7-Dihydro-3-[(2-hydroxyethyl)thio]-6,6-dimethyl-1-(2-thiazolyl)benzo[c]thiophen-4(5H)-one, TB 21007, TB-21007
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C15H17NO2S3
CAS Number:
Molecular Weight:
339.50
UNSPSC 코드:
51111800
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 2 mg/mL, clear
저장 온도
2-8°C
SMILES string
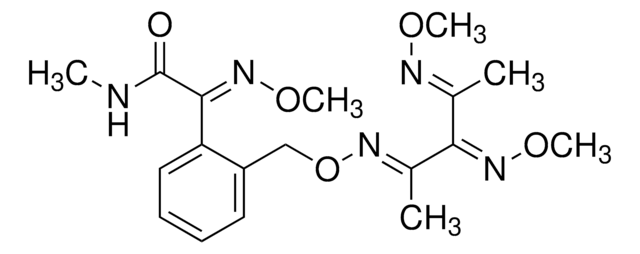
O=C1C2=C(SCCO)SC(C3=NC=CS3)=C2CC(C1)(C)C
InChI
1S/C15H17NO2S3/c1-15(2)7-9-11(10(18)8-15)14(20-6-4-17)21-12(9)13-16-3-5-19-13/h3,5,17H,4,6-8H2,1-2H3
InChI key
QILRYFCEXLFIDS-UHFFFAOYSA-N
생화학적/생리학적 작용
Gamma-aminobutyric acid type A receptor GABA(A) alpha 5 (α5) subtype-selective inverse agonist/negative allosteric modulator (NAM) in vitro and in vivo.
TB21007 is a gamma-aminobutyric acid type A receptor (GABAA) alpha 5 (α5) subtype-selective (αβ3γ2 Ki in nM = 1.6/α5, 20/α1, 16/α2, 20/α3) negative allosteric modulator (NAM)/inverse agonist (% inhibition at 100 nM = 51/α5, 21/α1, 1/α2, 3/α3, using L(tk-) fibroblast expressing β3γ2 with respective α subunit). TB21007 enhances cognitive performance in a hippocampal-dependent memory task in vivo (0.3 mg/kg i.p. in rats; delayed ‘matching-to-place′ Morris water maze test) without the convulsant or proconvulsant activity (3 mg/kg i.p. in mice) observed with nonselective inverse agonists.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Mark S Chambers et al.
Journal of medicinal chemistry, 46(11), 2227-2240 (2003-05-16)
In pursuit of a GABA(A) alpha5-subtype-selective inverse agonist to enhance cognition, a series of 6,7-dihydro-2-benzothiophen-4(5H)-ones has been identified as a novel class of GABA(A) receptor ligands. These thiophenes have higher binding affinity for the GABA(A) alpha5 receptor subtype compared to
K McEown et al.
Neuroscience, 252, 169-177 (2013-08-22)
Temporary neuronal inactivation of the ventral hippocampus with the GABAA agonist muscimol suppresses unconditioned fear behavior (anxiety) but inactivation of the dorsal hippocampus does not. On the other hand, inactivating the dorsal hippocampus disrupts fear memory, while inactivating the ventral
Ming Teng Koh et al.
Neurobiology of aging, 91, 1-4 (2020-04-03)
Numerous aging studies have identified a shift in the excitatory/inhibitory (E/I) balance with heightened hippocampal neural activity associated with age-related memory impairment across species, including rats, monkeys, and humans. Neurobiological investigations directed at the hippocampal formation have demonstrated that unimpaired
Yarim E De la Luz-Cuellar et al.
European journal of pharmacology, 858, 172443-172443 (2019-06-11)
The role of spinal α5 subunit-containing GABAA (α5-GABAA) receptors in chronic pain is controversial. The purpose of this study was to investigate the participation of spinal α5-GABAA receptors in the reserpine-induced pain model. Reserpine administration induced tactile allodynia and muscle
Ming Teng Koh et al.
Neuropharmacology, 64, 145-152 (2012-06-27)
A condition of excess activity in the hippocampal formation is observed in the aging brain and in conditions that confer additional risk during aging for Alzheimer's disease. Compounds that act as positive allosteric modulators at GABA(A) α5 receptors might be
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![E-64 N-[N-(L-3-Trans-carboxirane-2-carbonyl)-L-leucyl]-agmatine](/deepweb/assets/sigmaaldrich/product/structures/168/240/c77e3f6a-6709-4d17-b774-b850e8a54a51/640/c77e3f6a-6709-4d17-b774-b850e8a54a51.png)