추천 제품
Grade
analytical standard
Quality Level
CofA
current certificate can be downloaded
포장
ampule of 1000 mg
기술
HPLC: suitable
gas chromatography (GC): suitable
bp
128-130 °C/16 mmHg (lit.)
mp
32-38 °C (lit.)
density
2.382 g/mL at 25 °C (lit.)
응용 분야
environmental
형식
neat
저장 온도
2-30°C
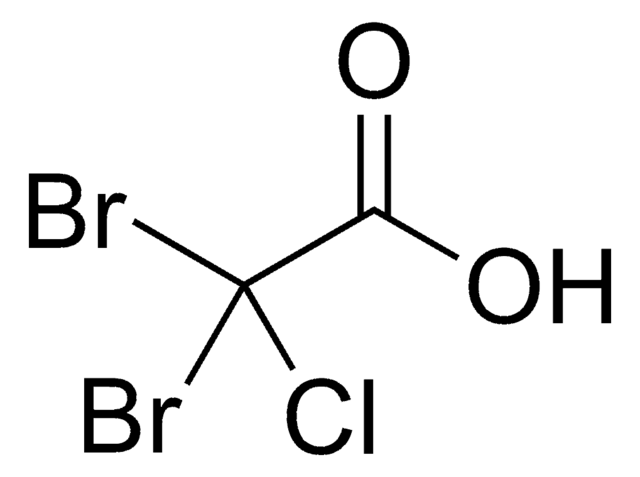
SMILES string
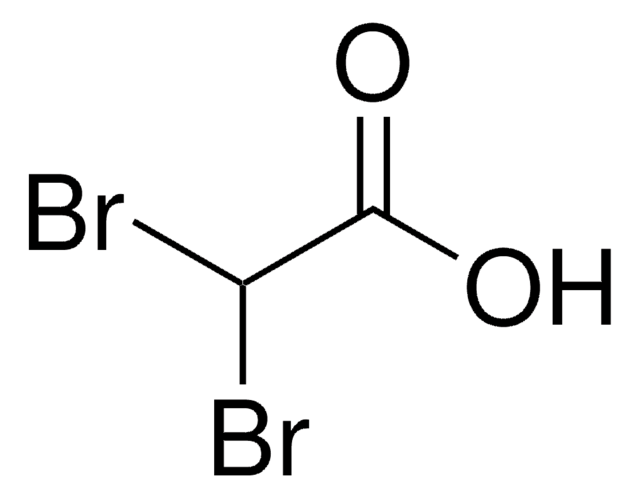
OC(=O)C(Br)Br
InChI
1S/C2H2Br2O2/c3-1(4)2(5)6/h1H,(H,5,6)
InChI key
SIEILFNCEFEENQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dibromoacetic acid belongs to the category of haloacetic acids (HAAs) which are a class of disinfection by-products produced in water.
애플리케이션
Dibromoacetic acid (Br2CHCOOH, CAS Number 631-64-1) may be used as an analytical reference standard for the determination of the analyte in aqueous samples by various chromatographic techniques.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Applications of ion chromatography with electrospray mass spectrometric detection to the determination of environmental contaminants in water.
Roehl R, et al.
Journal of Chromatography A, 956(1-2), 245-254 (2002)
Determination of haloacetic acids in aqueous environments by solid-phase extraction followed by ion-pair liquid chromatography-electrospray ionization mass spectrometric detection.
Loos R and Barcelo D
Journal of Chromatography A, 938(1-2), 45-55 (2001)
Determination of haloacetic acids in water by acidic methanol esterification-GC-ECD method.
Nikolaou AD, et al.
Water Research, 36(4), 1089-1094 (2002)
Determination of TOCl, TOBr and TOI in drinking water by pyrolysis and off-line ion chromatography.
Hua G and Reckhow DA
Analytical and Bioanalytical Chemistry, 384(2), 495-504 (2006)
Lianhui Tao et al.
Toxicological sciences : an official journal of the Society of Toxicology, 82(1), 62-69 (2004-09-03)
Dibromoacetic acid (DBA) is a drinking water disinfection by-product. Its analogs, dichloroacetic acid (DCA) and trichloroacetic acid (TCA), are liver carcinogens in rodents. We evaluated the ability of DBA to cause DNA hypomethylation, glycogen accumulation, and peroxisome proliferation that are
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.