1082300
USP
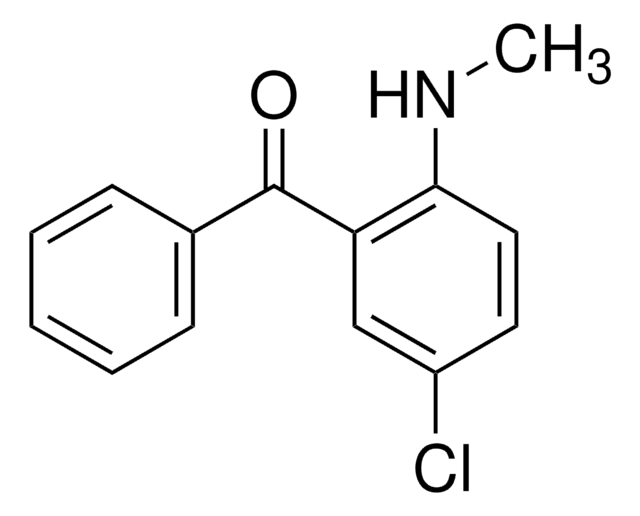
Butoconazole nitrate
United States Pharmacopeia (USP) Reference Standard
동의어(들):
(±)-1-[4-(4-Chlorophenyl)-2-[(2,6-dichlorophenyl)thio]butyl]-1H-imidazole mononitrate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H17Cl3N2S · HNO3
CAS Number:
Molecular Weight:
474.79
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
butoconazole
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
ClC1=C(C(Cl)=CC=C1)SC(CCC2=CC=C(Cl)C=C2)CN3C=CN=C3.O[N+]([O-])=O
InChI
1S/C19H17Cl3N2S.HNO3/c20-15-7-4-14(5-8-15)6-9-16(12-24-11-10-23-13-24)25-19-17(21)2-1-3-18(19)22;2-1(3)4/h1-5,7-8,10-11,13,16H,6,9,12H2;(H,2,3,4)
InChI key
ZHPWRQIPPNZNML-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Butoconazole nitrate (BN) is a imidazole used as an antifungal agent.
애플리케이션
Butoconazole nitrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monograph such as Butoconazole Nitrate Vaginal Cream.
Also, for use with USP monograph such as Butoconazole Nitrate Vaginal Cream.
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Meng-meng Jia et al.
Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban, 34(3), 431-436 (2014-06-19)
A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated for the determination of butoconazole in human plasma. Human plasma samples of 0.2 μL were pretreated by a single step protein precipitation procedure and analyzed using a high performance
J B Jacobson et al.
Acta obstetricia et gynecologica Scandinavica, 64(3), 241-244 (1985-01-01)
The continuously increasing incidence of vulvovaginal candidiasis necessitated a search for novel therapeutic modalities. Butoconazole nitrate (BN) a new imidazole, has been singled out for clinical studies since, in experimental vaginal candidiasis, it proved more effective than either miconazole nitrate
Larry S Seidman et al.
Infectious diseases in obstetrics and gynecology, 13(4), 197-206 (2005-12-13)
It is estimated that as many as 13 million cases of vulvovaginal infection occur in the United States annually, the majority of which are the result of Candida albicans infection. The symptoms of vulvovaginal infections are often painful and distressing
Ling Zhi Heng et al.
Singapore medical journal, 53(12), e269-e271 (2012-12-27)
Vulvo-vaginal candidiasis (VVC) is a common infection among women. 5% of women with acute infection experience recurrent vulvo-vaginal candidiasis (RVVC). There is currently no optimal or recommended regime for RVVC. Although antifungal agents, such as imidazoles, have been successfully used
A Nikolov et al.
Akusherstvo i ginekologiia, 46(9), 23-26 (2008-07-23)
To study the therapeutic application of Gynazol in cases of recurrent vaginal Candida infections during the last trimester of pregnancy. 28 pregnant women in third trimester with recurrent vaginal Candida infection were included in the study. All cases were treated
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.