103012
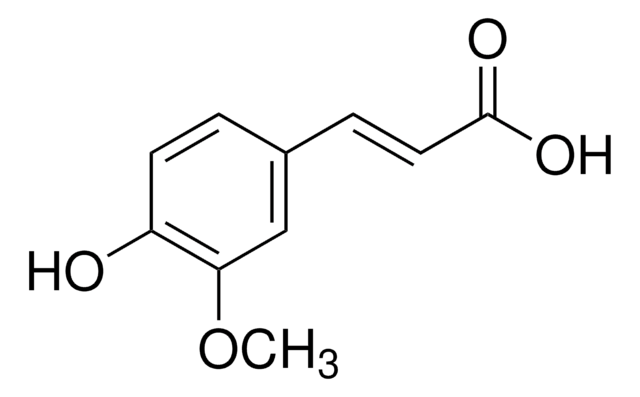
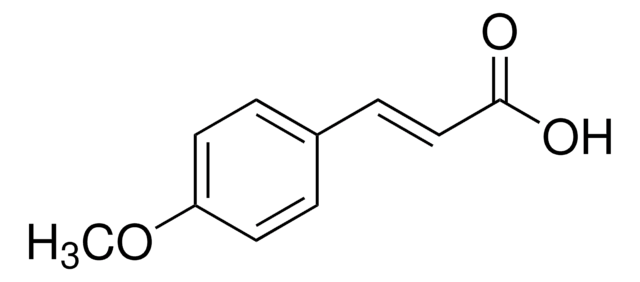
3-Hydroxy-4-methoxycinnamic acid, predominantly trans
97%
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H3(OCH3)CH=CHCO2H
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
230 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(\C=C\C(O)=O)cc1O
InChI
1S/C10H10O4/c1-14-9-4-2-7(6-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+
InChI key
QURCVMIEKCOAJU-HWKANZROSA-N
Related Categories
General description
3-Hydroxy-4-methoxycinnamic acid, predominantly trans is available as white crystals. 3-Hydroxy-4-methoxycinnamic acid is isolated from the aerial part of Artemisia capillaris, Chinese medicinal plant. It is a component of chinese herbal medicine used for a pain killer and stomachic. It is an efficient acetylcholine inhibitor. 3-Hydroxy-4-methoxycinnamic acid is bioactive metabolite of Spilanthes acmella Murr. It increases the resistance of low-density lipoprotein (LDL) to oxidation.
Application
3-Hydroxy-4-methoxycinnamic acid was used in the synthesis of tranilast and various tranilast analogs (cinnamoyl anthranilates) by genetically engineered Saccharomyces cerevisiae yeast strain. It was also used in the synthesis of glycoside compounds by undergoing glycosidation.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Supaluk Prachayasittikul et al.
Molecules (Basel, Switzerland), 14(2), 850-867 (2009-03-04)
Spilanthes acmella Murr. (Compositae) has been used as a traditional medicine for toothache, rheumatism and fever. Its extracts had been shown to exhibit vasorelaxant and antioxidant activities. Herein, its antimicrobial, antioxidant and cytotoxic activities were evaluated. Agar dilution method assays
I-Min Liu et al.
The Journal of pharmacology and experimental therapeutics, 307(3), 1196-1204 (2003-09-17)
We investigated the mechanism(s) by which isoferulic acid lowers plasma glucose levels in streptozotocin-induced diabetic rats (STZ-diabetic rats). In STZ-diabetic rats, isoferulic acid dose dependently lowered plasma glucose concentrations and increased plasma beta-endorphin-like immunoreactivity (BER). Both of these effects of
Jonathan M Hodgson et al.
The British journal of nutrition, 91(2), 301-306 (2004-02-06)
Tea and coffee are rich in polyphenols with a variety of biological activities. Many of the demonstrated activities are consistent with favourable effects on the risk of chronic diseases. 4-O-methylgallic acid (4OMGA) and isoferulic acid are potential biomarkers of exposure
Adriana Farah et al.
The Journal of nutrition, 138(12), 2309-2315 (2008-11-22)
Chlorogenic acids (CGA) are cinnamic acid derivatives with biological effects mostly related to their antioxidant and antiinflammatory activities. Caffeoylquinic acids (CQA) and dicaffeoylquinic acids (diCQA) are the main CGA found in nature. Because green coffee is a major source of
A R Rechner et al.
Free radical biology & medicine, 30(11), 1213-1222 (2001-05-23)
The purpose of this study was to investigate biomarkers of the bioavailability and metabolism of hydroxycinnamate derivatives through the determination of the pharmacokinetics of their urinary elimination and identification of the metabolites excreted. Coffee was used as a rich source
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service