106399
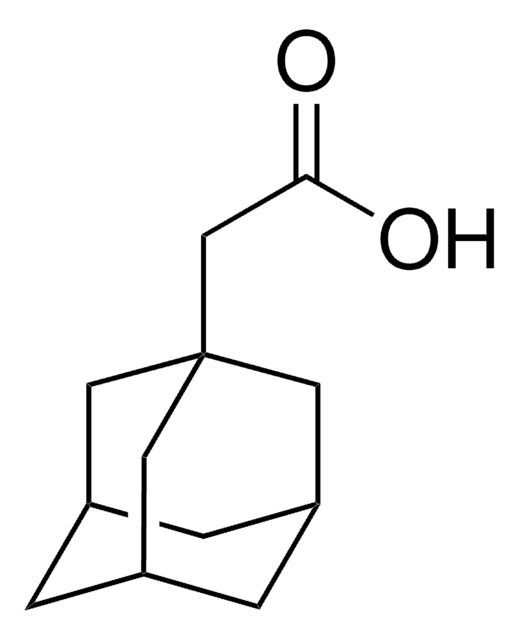
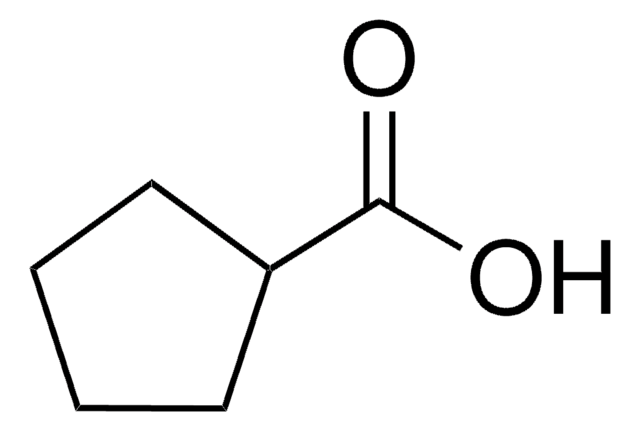
1-Adamantanecarboxylic acid
99%
Synonym(s):
Adamantane carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O2
CAS Number:
Molecular Weight:
180.24
Beilstein/REAXYS Number:
1910637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
mp
172-174 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C11H16O2/c12-10(13)11-4-7-1-8(5-11)3-9(2-7)6-11/h7-9H,1-6H2,(H,12,13)/t7-,8+,9-,11-
InChI key
JIMXXGFJRDUSRO-KJZNFTALSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Adamantanecarboxylic acid can be used as:
- A stabilizer in the synthesis of monodisperse, highly crystalline CoPt3 nanoparticles and porous platinum nanoparticles.
- An additive in polycondensation reactions to yield conjugated polymers as possible optoelectronic materials.
- An additive in the allylic substitution reaction, which is catalyzed by palladium in an aqueous medium.
Biochem/physiol Actions
1-Adamantanecarboxylic acid undergoes complexation reactions with cyclohexaamylose. It is an inhibitor of phenyl ester hydrolysis of cycloheptaamylose.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiaopeng Han et al.
Journal of chromatographic science, 54(8), 1460-1465 (2016-06-01)
An efficient extraction of doxorubicin (Dox) from homemade stealth hyalurionic acid (HA)-based nanoparticles (NPs) in rat plasma could not be performed by previously published methods. Therefore, we attempted to establish the novel NPs-breaking and UPLC-MS-MS method for evaluating the pharmacokinetic
Ronit Freeman et al.
Nano letters, 9(5), 2073-2076 (2009-04-10)
Beta-cyclodextrin (beta-CD)-functionalized CdSe/ZnS quantum dots (QDs) are used for optical sensing and chiroselective sensing of different substrates using a fluorescence resonance energy transfer (FRET) or an electron transfer (ET) mechanisms. The FRET between the QDs and Rhodamine B incorporated in
Palladium-catalyzed, carboxylic acid-assisted allylic substitution of carbon nucleophiles with allyl alcohols as allylating agents in water
Manabe K and Kobayashi S
Organic Letters, 5(18), 3241-3244 (2003)
Synthesis of porous platinum nanoparticles.
Xiaowei Teng et al.
Small (Weinheim an der Bergstrasse, Germany), 2(2), 249-253 (2006-12-29)
Fang-Fei Zhu et al.
Food chemistry, 257, 382-387 (2018-04-07)
Amantadine (AMD), a banned antiviral veterinary drug, is still being abused. This study developed a novel enzyme linked immunosorbent assay for the colorimetric detection of AMD involving DNA hybridization reaction and non-crosslinking gold nanoparticles (AuNPs) aggregation. Accordingly, the Primer 1-AuNPs-anti-AMD
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service