All Photos(2)
About This Item
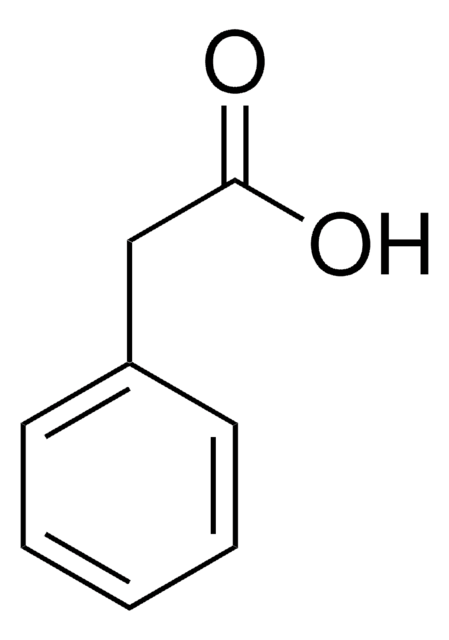
Linear Formula:
C6H5CH2CO2CH3
CAS Number:
Molecular Weight:
150.17
Beilstein/REAXYS Number:
878795
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
assay
≥99%
refractive index
n20/D 1.503 (lit.)
bp
218 °C (lit.)
density
1.066 g/mL at 20 °C (lit.)
SMILES string
COC(=O)Cc1ccccc1
InChI
1S/C9H10O2/c1-11-9(10)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3
InChI key
CRZQGDNQQAALAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl phenylacetate was used as model compound for partition coefficient measurement experiments.
Biochem/physiol Actions
Methyl phenylacetate undergoes decomposition on photolysis in methanol. Methyl phenylacetate acts as acylating agent and causes the enantioselective acylation of beta-lactam intermediate using penicillin G amidase. Methyl phenylacetate is the starting material in manufacture of synthetic perfumes.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
211.1 °F - closed cup
flash_point_c
99.5 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yichen Cao et al.
Journal of pharmaceutical sciences, 93(11), 2768-2779 (2004-09-25)
The ability to predict drug solubility and partitioning in triglyceride solvents from the chemical structures of the solute and the triglyceride would be highly useful in drug formulation development and in screening drug candidates for lipid solubility and possibly drug
Enantioselective acylation of a beta-lactam intermediate in the synthesis of loracarbef using penicillin G amidase.
Zmijewski Jr MJ, et al.
Tetrahedron Letters, 32(13), 1621-1622 (1991)
Photolysis of phenylacetic acid and methyl phenylacetate in methanol.
Meiggs TO and Miller SI.
Journal of the American Chemical Society, 94(6), 1989-1996 (1972)
Miriam Frida Karlsson et al.
Journal of agricultural and food chemistry, 57(13), 5903-5909 (2009-06-06)
The Guatemalan moth Tecia solanivora is an invasive pest of potato in Central and South America. The larvae infest potato tubers in the field as well as in storage facilities. The headspace of potato foliage and potato tubers was studied
Jonathan Slaughter et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(1), 167-175 (2016-10-30)
In investigating and seeking to mimic the reactivity of trimethylaluminium (TMA) with synthetic, ester-based lubricating oils, the reaction of methyl propionate 1 was explored with 1, 2 and 3 equivalents of the organoaluminium reagent. Spectroscopic analysis points to the formation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service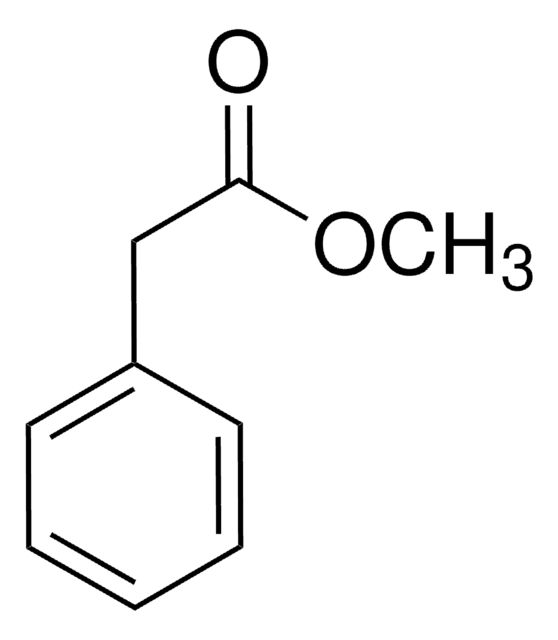

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)