131326
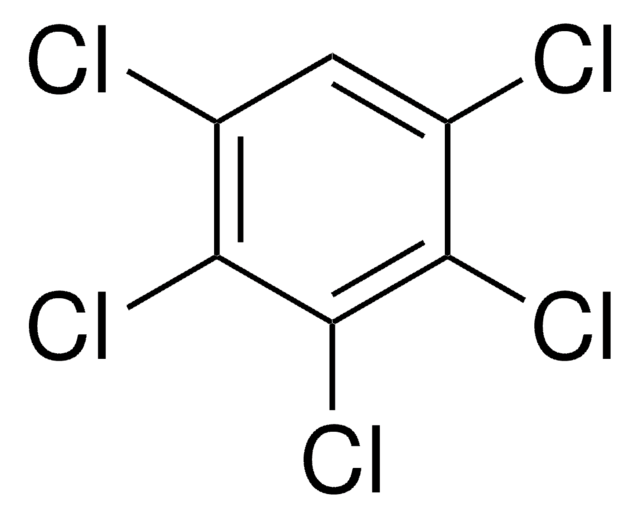
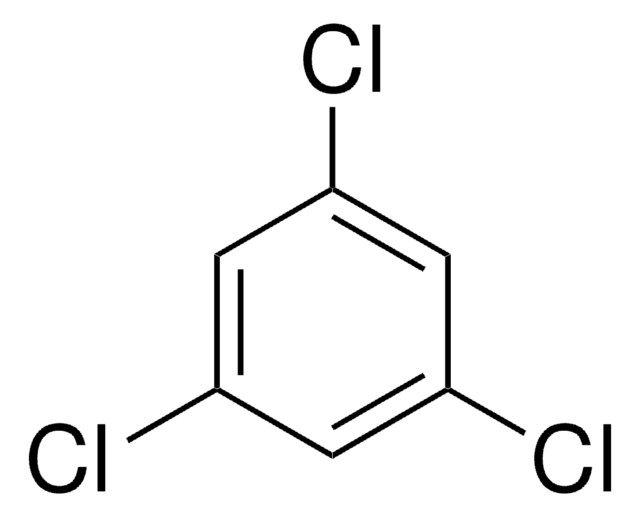
Pentachlorobenzene
96%
Synonym(s):
1,2,3,4,5-Pentachlorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HC6Cl5
CAS Number:
Molecular Weight:
250.34
Beilstein/REAXYS Number:
1911550
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
solid
bp
275-277 °C (lit.)
mp
84-87 °C (lit.)
density
1.609 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Clc1cc(Cl)c(Cl)c(Cl)c1Cl
InChI
1S/C6HCl5/c7-2-1-3(8)5(10)6(11)4(2)9/h1H
InChI key
CEOCDNVZRAIOQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Pentachlorobenzene was determined in the water samples by packed sorbent (MEPS) coupled directly to programmed temperature vaporizer-gas chromatography-mass spectrometry.
Application
Pentachlorobenzene was used to prepare tetrachlorobenzene by photolysis.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Sol. 1
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of bridgehead fluorides by fluorodeiodination.
Della EW and Head NJ. et al.
The Journal of Organic Chemistry, 57(10), 2850-2855 (1992)
Gloria Grueiro Noche et al.
Analytical and bioanalytical chemistry, 405(21), 6739-6748 (2013-06-20)
A fully automated method consisting of microextraction by packed sorbent (MEPS) coupled directly to programmed temperature vaporizer-gas chromatography-mass spectrometry (PTV-GC-MS) has been developed to determine the 12 chlorobenzene congeners (chlorobenzene; 1,2-, 1,3-, and 1,4-dichlorobenzene; 1,2,3-, 1,2,4-, and 1,3,5-trichlorobenzene; 1,2,3,4-, 1,2,3,5-
Daniel Carrizo et al.
Ecotoxicology and environmental safety, 71(1), 260-266 (2007-10-16)
The study of a population of 4-year-old children born between 1997 and 1999 in an urban area under strong inputs of pentachlorobenzene (PeCB) and hexachlorobenzene (HCB) suggested that the measured concentrations of pentachlorophenol (PCP) in serum may essentially result from
Ian John Allan et al.
Journal of environmental monitoring : JEM, 13(9), 2420-2426 (2011-07-27)
Thirty two polychlorinated biphenyl congeners (PCBs), hexachlorobenzene (HCB) and pentachlorobenzene (PeCB) were analysed in passive sampler extracts from surface water-exposed semipermeable membrane devices (SPMDs) and in bed sediment samples from a small urban watercourse, the River Alna (Oslo, Norway). Performance
N Martí et al.
Marine pollution bulletin, 62(3), 615-625 (2011-02-08)
A comprehensive study aimed at evaluating the occurrence, significance of concentrations and spatial distribution of priority pollutants (PPs) along the Comunidad Valenciana coastal waters (Spain) was carried out in order to fulfil the European Water Framework Directive (WFD). Additionally, PP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service