137448
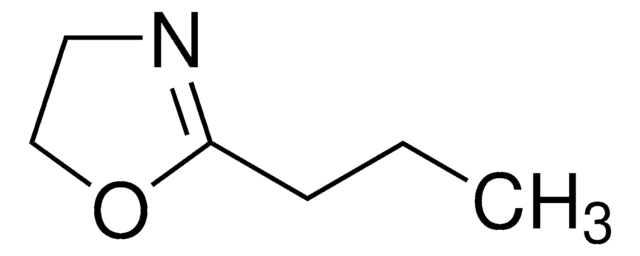
2-Methyl-2-oxazoline
98%
Synonym(s):
2-Methyloxazoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7NO
CAS Number:
Molecular Weight:
85.10
Beilstein/REAXYS Number:
104227
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
109.5-110.5 °C (lit.)
density
1.005 g/mL at 25 °C (lit.)
SMILES string
CC1=NCCO1
InChI
1S/C4H7NO/c1-4-5-2-3-6-4/h2-3H2,1H3
InChI key
GUXJXWKCUUWCLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Methyl-2-oxazoline undergoes polymerization with 2-butyl-2-oxazoline to form poly(2-oxazoline) block copolymer. It undergoes cationic ring-opening polymerization with 2-(dec-9-enyl)-2-oxazoline to yield copoly(2-oxazoline)s.
Application
2-Methyl-2-oxazoline was used in cationic polymerization of a series of linear 2-alkyl-2-oxazolines under microwave irradiation.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted synthesis and properties of a series of poly (2-alkyl-2-oxazoline) s.
Hoogenboom R, et al.
Designed Monomers and Polymers, 8(6), 659-671 (2005)
Giulia Morgese et al.
ACS nano, 11(3), 2794-2804 (2017-03-09)
Osteoarthritis leads to an alteration in the composition of the synovial fluid, which is associated with an increase in friction and the progressive and irreversible destruction of the articular cartilage. In order to tackle this degenerative disease, there has been
Yunjiao Che et al.
Soft matter, 16(29), 6733-6742 (2020-06-27)
We report a novel double cross-linked hydrogel system based on polyacrylamide and poly(2-methyl-2-oxazoline) (PMOXA) network chains, as well as on supramolecular host-guest interactions with on-demand tailored mechanical properties. Well-defined vinyl-bearing PMOXA macromonomers, functionalized with either β-cyclodextrin units (β-CD-PMOXA) or adamantane
Yalin Zhang et al.
Talanta, 150, 375-387 (2016-02-04)
Nitrogen-rich melamine (66% by mass) had been found to be illegally adulterated to milk products and animal feed in order to increase the apparent protein content, and ingestion of melamine may lead to the formation of kidney stones. Hence, the
Pavel Sťahel et al.
Polymers, 11(12) (2019-12-18)
Polyoxazolines are a new promising class of polymers for biomedical applications. Antibiofouling polyoxazoline coatings can suppress bacterial colonization of medical devices, which can cause infections to patients. However, the creation of oxazoline-based films using conventional methods is difficult. This study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service