144711
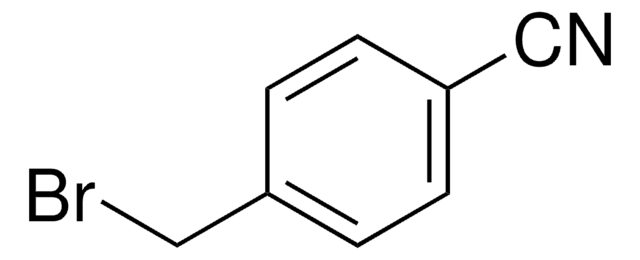
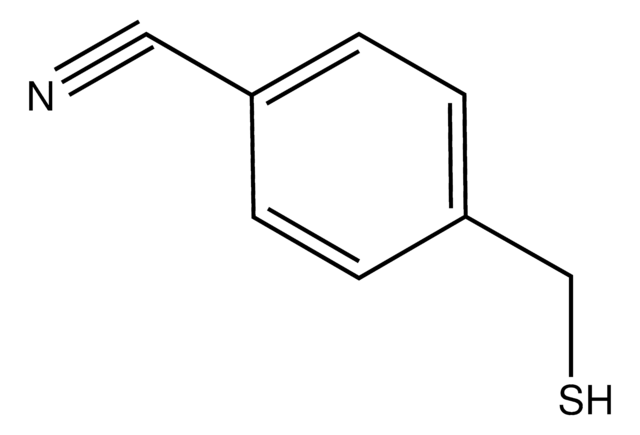
2-(Bromomethyl)benzonitrile
≥97%
Synonym(s):
α-Bromo-o-tolunitrile, 2-Cyanobenzyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2C6H4CN
CAS Number:
Molecular Weight:
196.04
Beilstein/REAXYS Number:
742605
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥97%
form
solid
mp
72-74 °C (lit.)
functional group
bromo
nitrile
SMILES string
BrCc1ccccc1C#N
InChI
1S/C8H6BrN/c9-5-7-3-1-2-4-8(7)6-10/h1-4H,5H2
InChI key
QGXNHCXKWFNKCG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-(Bromomethyl)benzonitrile reacts with 2H-tetrazole in the presence of KOH to yield 4-[(2H-tetra-zol-2-yl)meth-yl]benzonitrile. It undergoes base-promoted condensation reaction with homophthalic anhydride to yield 6,11-dihydro-5H-indeno[1,2-c]isoquinolin-5-one.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Prakash G Jagtap et al.
Organic letters, 7(9), 1753-1756 (2005-04-23)
[reaction: see text] The synthesis of 6,11-dihydro-5H-indeno[1,2-c]isoquinolin-5-ones from the base-promoted condensation reaction of homophthalic anhydride and 2-(bromomethyl)-benzonitrile and a convenient method for the synthesis of indolo[3,2-c]isoquinolinones are described.
Zheng Xing et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o445-o445 (2008-01-01)
The title compound, C(9)H(7)N(5), was synthesized by reaction of 4-(bromomethyl)benzonitrile and 2H-tetrazole in the presence of KOH. The relative orientation of the planar tetra-zole ring and the methyl-benzonitrile moiety is (-)-anti-clinal. The crystal packing is dominated by van der Waals
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service