All Photos(2)
About This Item
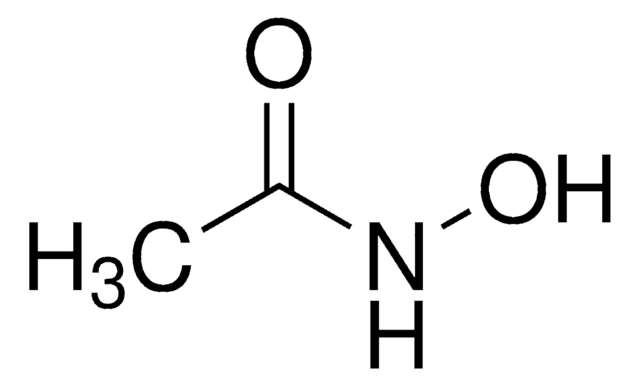
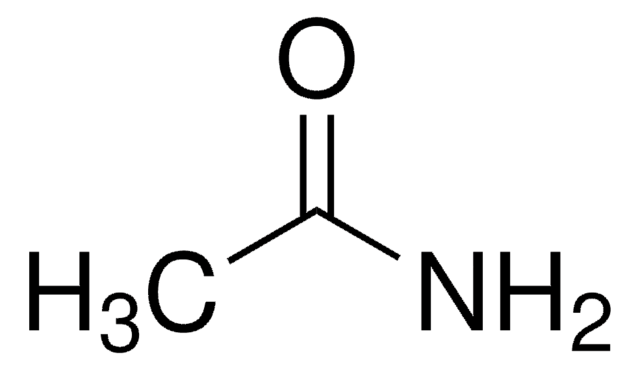
Linear Formula:
CH3CONHOH
CAS Number:
Molecular Weight:
75.07
Beilstein/REAXYS Number:
1739019
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
88-90 °C (lit.)
functional group
amine
SMILES string
CC(NO)=O
InChI
1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4)
InChI key
RRUDCFGSUDOHDG-UHFFFAOYSA-N
Gene Information
human ... CA2(760) , MMP3(4314)
Looking for similar products? Visit Product Comparison Guide
General description
Acetohydroxamic acid is a potent inhibitor of bacterial urease activity and reduces urinary ammonia levels. 2-Acetohydroxamic acid loaded floating microspheres forms an efficient drug delivery system for the treatment of Helicobacter pylori.
Application
Acetohydroxamic acid was used:
- to study the mechanism of complexation of iron (III) with acetohydroxamic acid
- to study the inhibitory mechanism of lansoprazole and omeprazole on Helicobacter pyloni
- in urease inhibition studies
- for in situ generation of nitrosocarbonylmethane as a Diels-Alder dienophile
Used in urease inhibition studies and for in situ generation of nitrosocarbonylmethane as a Diels-Alder dienophile.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mechanism of iron (III) complex formation. Activation volumes for the complexation of the iron (III) ion with thiocyanate ion and acetohydroxamic acid.
Funahashi S, et al.
Inorganic Chemistry, 22(14), 2070-2073 (1983)
R B Umamaheshwari et al.
The Journal of pharmacy and pharmacology, 55(12), 1607-1613 (2004-01-24)
This investigation is part of our ongoing effort to develop effective drug delivery systems for the treatment of Helicobacter pylori infection using polycarbonate (PC) floating microspheres as drug carriers. In an effort to augment the anti-H. pylori effect of acetohydroxamic
Chem. Abstr., 116, 124530f-124530f (1992)
D P Griffith et al.
The Journal of urology, 140(2), 318-324 (1988-08-01)
Acetohydroxamic acid is known to inhibit bacterial urease activity, thus, reducing urinary ammonia levels. A double-blind placebo-controlled clinical trial of acetohydroxamic acid was conducted at 12 Veterans Administration spinal cord injury units. A total of 210 male spinal cord injury
Journal of the Chemical Society. Perkin Transactions 1, 1001-1001 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service