185922
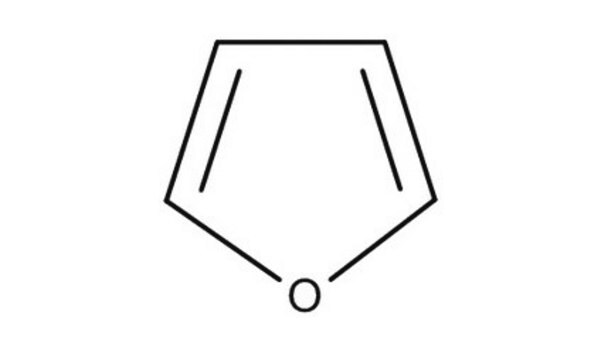
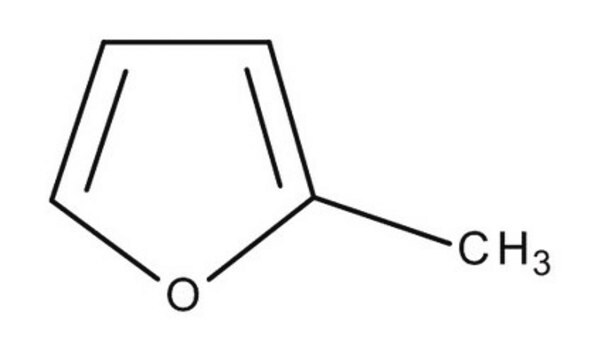
Furan
≥99%
Synonym(s):
1,4-Epoxybuta-1,3-diene, Divinylene oxide, Oxacyclopentadiene, Oxole, Tetrole
About This Item
Recommended Products
vapor density
2.35 (vs air)
Quality Level
vapor pressure
1672 mmHg ( 55 °C)
31.66 psi ( 55 °C)
493 mmHg ( 20 °C)
9.22 psi ( 20 °C)
assay
≥99%
form
liquid
contains
0.025 wt. % BHT as inhibitor
expl. lim.
14.3 %
refractive index
n20/D 1.421 (lit.)
bp
32 °C/758 mmHg (lit.)
solubility
alcohols: freely soluble
diethyl ether: freely soluble
water: insoluble
density
0.936 g/mL at 25 °C (lit.)
shipped in
wet ice
storage temp.
2-8°C
SMILES string
c1ccoc1
InChI
1S/C4H4O/c1-2-4-5-3-1/h1-4H
InChI key
YLQBMQCUIZJEEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Preparation of the starting material required for the synthesis of calix[6]pyrrole.
- To investigate the kinetics and mechanism of reactions of chlorine atoms with volatile organic compounds.
- Catalytic transformation of furan to aromatics and olefins.
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 1B - Flam. Liq. 1 - Muta. 2 - Skin Irrit. 2 - STOT RE 2
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-32.8 °F - closed cup
flash_point_c
-36 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service