208264
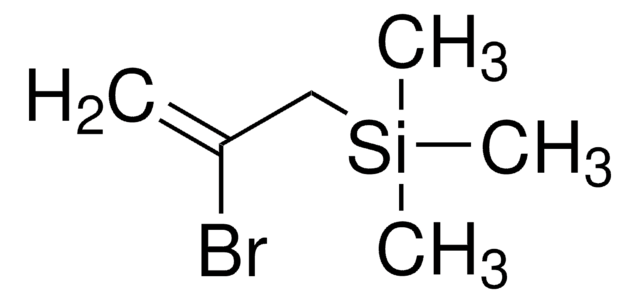
Allyltrimethylsilane
98%
Synonym(s):
3-(Trimethylsilyl)propene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2C=CHCH2Si(CH3)3
CAS Number:
Molecular Weight:
114.26
Beilstein/REAXYS Number:
906755
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
84-88 °C (lit.)
density
0.719 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)CC=C
InChI
1S/C6H14Si/c1-5-6-7(2,3)4/h5H,1,6H2,2-4H3
InChI key
HYWCXWRMUZYRPH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Allyltrimethylsilane is a general reagent to introduce allyl groups across acid chlorides, aldehydes, ketones, iminium ions, enones, and for cross-coupling with other carbon electrophiles. It is used as a reagent in Hosomi−Sakurai reaction.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
60.8 °F - closed cup
flash_point_c
16 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ming Yu et al.
Organic letters, 5(24), 4639-4640 (2003-11-25)
[reaction: see text] Direct allylation of glycal-derived donor-acceptor cyclopropanes has been achieved with TiCl(4) activation followed by addition of allyltrimethylsilane. The alpha diastereomer is the major product, with selectivities ranging from 3:1 to 10:1 and yields around 80%.
A general and efficient FeCl3-catalyzed nucleophilic substitution of propargylic alcohols
Zhan Z, et al.
The Journal of Organic Chemistry, 71(21), 8298-8301 (2006)
Ionic liquid-promoted, highly regioselective heck arylation of electron-rich olefins by aryl halides
Mo J, et al.
Journal of the American Chemical Society, 127(2), 751-760 (2005)
Roman Schowner et al.
Angewandte Chemie (International ed. in English), 59(2), 951-958 (2019-11-28)
The origin of hydroxyl group tolerance in neutral and especially cationic molybdenum imido alkylidene N-heterocyclic carbene (NHC) complexes has been investigated. A wide range of catalysts was prepared and tested. Most cationic complexes can be handled in air without difficulty
Control of alpha/beta stereoselectivity in Lewis acid promoted C-glycosidations using a controlling anomeric effect based on the conformational restriction strategy.
Satoru Tamura et al.
Angewandte Chemie (International ed. in English), 42(9), 1021-1023 (2003-03-05)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service