All Photos(1)
About This Item
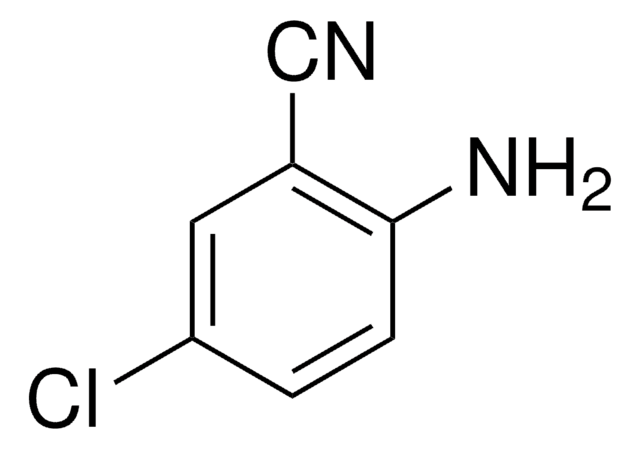
Linear Formula:
H2NC6H3(CH3)CN
CAS Number:
Molecular Weight:
132.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
92-95 °C (lit.)
functional group
nitrile
SMILES string
Cc1ccc(C#N)c(N)c1
InChI
1S/C8H8N2/c1-6-2-3-7(5-9)8(10)4-6/h2-4H,10H2,1H3
InChI key
LGNVAEIITHYWCG-UHFFFAOYSA-N
Application
2-Amino-4-methylbenzonitrile was used in the synthesis of:
- 7-methyl-4-(phenylamino)quinazoline-2(1H)-selone
- racemic aminoquinolines, potential acetylcholinesterase (AChE) inhibitors
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 4-(Phenylamino) quinazoline-2 (1H)-selones and Diselenides from Isoselenocyanates: Dimroth Rearrangement of an Intermediate.
Atanassov PK, et al.
Helvetica Chimica Acta, 87(7), 1873-1877 (2004)
P Camps et al.
Journal of medicinal chemistry, 42(17), 3227-3242 (1999-08-28)
Eleven new 12-amino-6,7,10,11-tetrahydro-7, 11-methanocycloocta[b]quinoline derivatives [tacrine (THA)-huperzine A hybrids, rac-21-31] have been synthesized as racemic mixtures and tested as acetylcholinesterase (AChE) inhibitors. For derivatives unsubstituted at the benzene ring, the highest activity was obtained for the 9-ethyl derivative rac-20, previously
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service