All Photos(1)
About This Item
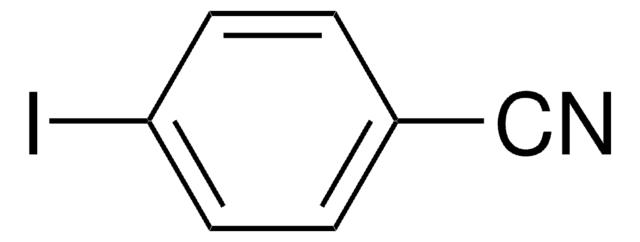
Linear Formula:
FC6H4I
CAS Number:
Molecular Weight:
222.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.584 (lit.)
bp
77-78 °C/19 mmHg (lit.)
density
1.89 g/mL at 25 °C (lit.)
functional group
fluoro
iodo
SMILES string
Fc1cccc(I)c1
InChI
1S/C6H4FI/c7-5-2-1-3-6(8)4-5/h1-4H
InChI key
VSKSBSORLCDRHS-UHFFFAOYSA-N
General description
3-Fluoroiodobenzene participates in palladium-catalyzed hydroarylation of arylpropiolamides.
Application
3-Fluoroiodobenzene was used to prepare methyl 4-iodobenzo[b]thiophene-2-carboxylate, key intermediate for the synthesis of 4-substituted benzo[b]thiophene-2-carboxamidines.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
152.6 °F - closed cup
flash_point_c
67 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M J Towle et al.
Cancer research, 53(11), 2553-2559 (1993-06-01)
Urokinase-type plasminogen activator (uPA) is an important mediator of cellular invasiveness. Specifically, cell surface receptor-bound uPA activates plasminogen to the potent general protease plasmin, which then degrades extracellular matrix or basement membrane either directly or via proteolytic activation of latent
A regio-and stereocontrolled method for preparing 3, 3-diarylacrylamides.
Hay LA and Mitchell D.
Tetrahedron Letters, 38(37), 6533-6536 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service