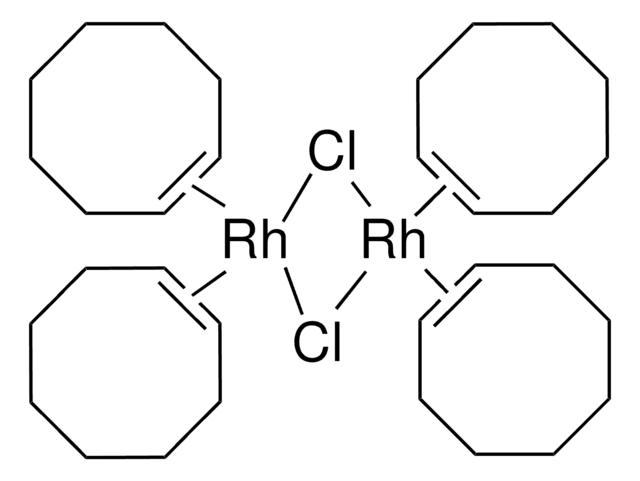
227951
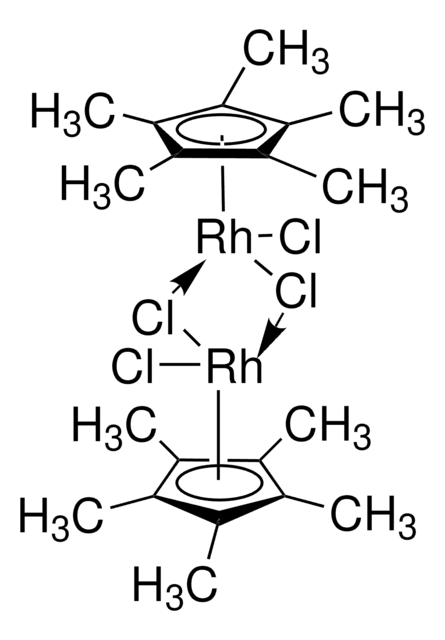
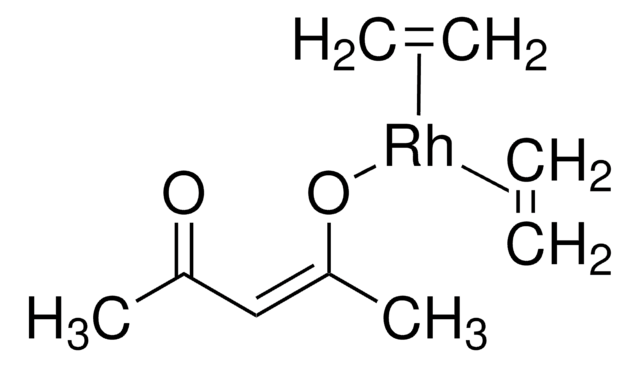
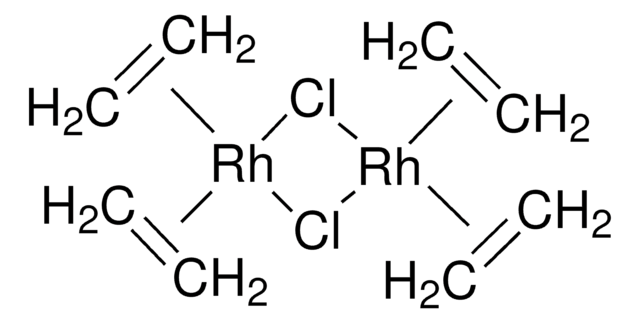
Chloro(1,5-cyclooctadiene)rhodium(I) dimer
98%
Synonym(s):
1,5-Cyclooctadienerhodium(I) chloride dimer, Bis(1,5-cyclooctadiene)dirhodium(I) dichloride, Di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]dirhodium, Rhodium(I) chloride 1,5-Cyclooctadiene complex dimer, [Rh(COD)Cl]2
About This Item
Recommended Products
Quality Level
assay
98%
form
solid
reaction suitability
core: rhodium
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
243 °C (dec.) (lit.)
greener alternative category
SMILES string
Cl[Rh].Cl[Rh].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.2ClH.2Rh/c2*1-2-4-6-8-7-5-3-1;;;;/h2*1-2,7-8H,3-6H2;2*1H;;/q;;;;2*+1/p-2/b2*2-1-,8-7-;;;;
InChI key
QSUDXYGZLAJAQU-MIXQCLKLSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
Efficient and Selective Catalysts for Asymmetric Synthesis
Rhodium-Catalyzed Oxygenative Addition to Terminal Alkynes for the Synthesis of Esters, Amides, and Carboxylic Acids
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer 96%](/deepweb/assets/sigmaaldrich/product/structures/700/585/b2e5ae1d-2b88-42c8-a071-ef828d4a104c/640/b2e5ae1d-2b88-42c8-a071-ef828d4a104c.png)