234893
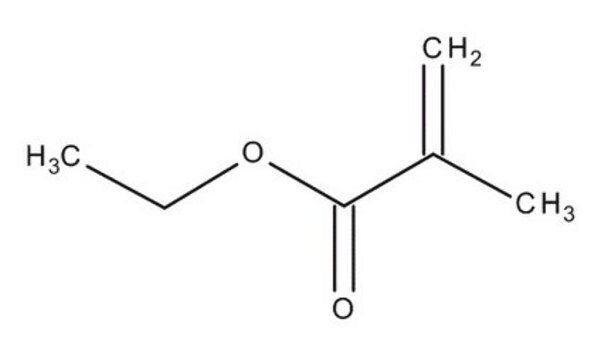
Ethyl methacrylate
contains 15-20 ppm monomethyl ether hydroquinone as inhibitor, 99%
Synonym(s):
2-Methyl-2-propenoic acid
Select a Size
Select a Size
About This Item
Recommended Products
vapor density
>3.9 (vs air)
vapor pressure
15 mmHg ( 20 °C)
assay
99%
form
liquid
autoignition temp.
771 °F
contains
15-20 ppm monomethyl ether hydroquinone as inhibitor
refractive index
n20/D 1.413 (lit.)
bp
118-119 °C (lit.)
density
0.917 g/mL at 25 °C (lit.)
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl methacrylate is a readily polymerizable monomer used for certain types of acrylic resins. The monomethyl ether hydroxyl quinone present in it is an inhibitor that prevents polymerization.
Application
- To synthesize artificial nanosized latexes of poly(styrene-co-methyl methacrylate) or poly(styrene-co-ethyl methacrylate), which are in producing drug-releasing films.
- In the production of additive-manufactured methacrylate-based resins used in dentistry.
- In the synthesis of a star-shaped block copolymer electrolyte for all-solid-state lithium batteries.
- In the synthesis of a copolymer used as a matrix for semiconductor nanoparticles, which is crucial for the formation of a stable matrix for the quantum dots-copolymer composite material used in optoelectronic applications.
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
66.2 °F - closed cup
flash_point_c
19 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![2-[3-(2H-Benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate 99%](/deepweb/assets/sigmaaldrich/product/structures/208/967/cf29567e-c125-41dc-b80a-66889fa1a679/640/cf29567e-c125-41dc-b80a-66889fa1a679.png)
