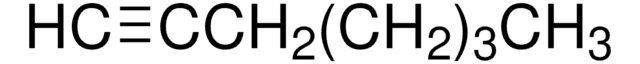All Photos(1)
About This Item
Linear Formula:
CH3(CH2)6C≡CH
CAS Number:
Molecular Weight:
124.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
16 mmHg ( 37.7 °C)
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.422 (lit.)
bp
150-151 °C (lit.)
mp
−50 °C (lit.)
density
0.757 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCC#C
InChI
1S/C9H16/c1-3-5-7-9-8-6-4-2/h1H,4-9H2,2H3
InChI key
OSSQSXOTMIGBCF-UHFFFAOYSA-N
Related Categories
General description
1-Nonyne reacts with thianthrene cation radical tetrafluoroborate to form trans-1,2-bis(5-thianthreniumyl)alkene tetrafluoroborate.
Application
1-Nonyne has been used in enantioselective synthesis of cladospolide B, C and (ent)-cladospolide D.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
93.2 °F - closed cup
flash_point_c
34 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paramashivappa Rangappa et al.
The Journal of organic chemistry, 70(24), 9764-9770 (2005-11-19)
[structure: see text] Thianthrene cation radical tetrafluoroborate (Th*+ BF4(-)) added to the terminal alkynes 1-pentyne, 1-hexyne, 1-heptyne, 1-octyne, 1-nonyne, and 1-decyne to form trans-1,2-bis(5-thianthreniumyl)alkene tetrafluoroborates (1-6). Similarly, addition of phenoxathiin cation radical tetrafluoroborate (PO*+ BF4(-)) to the same alkynes gave
Yalan Xing et al.
Organic letters, 11(5), 1107-1110 (2009-02-06)
The enantioselective synthesis of cladospolide B, C, and (ent)-cladospolide D has been achieved in 11-15 steps from 1-nonyne. The route relies upon an alkyne zipper reaction to relay an ynone and dienoate functional groups across a nine carbon fragment, which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service