All Photos(1)
About This Item
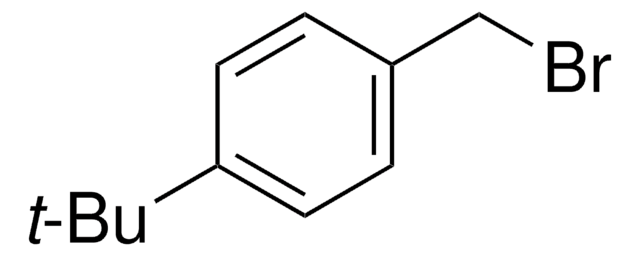
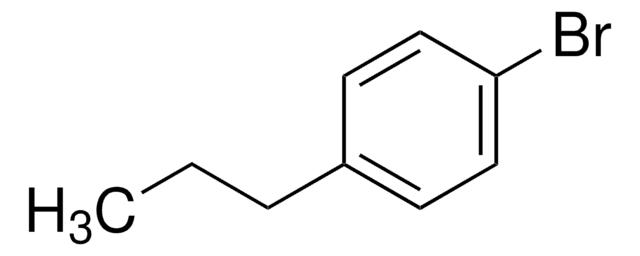
Linear Formula:
(CH3)3CC6H4Br
CAS Number:
Molecular Weight:
213.11
Beilstein/REAXYS Number:
1859117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
liquid
refractive index
n20/D 1.533 (lit.)
bp
80-81 °C/2 mmHg (lit.)
mp
13-16 °C (lit.)
density
1.229 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
CC(C)(C)c1ccc(Br)cc1
InChI
1S/C10H13Br/c1-10(2,3)8-4-6-9(11)7-5-8/h4-7H,1-3H3
InChI key
XHCAGOVGSDHHNP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Bromo-4-tert-butylbenzene undergoes lithium-bromide exchange reactions with n-butyllithium and tert-butyllithium at 0°C in various solvents.
Application
1-Bromo-4-tert-butylbenzene was used in the synthesis of 4-tert-butyl-phenylboronic acid, 1-deoxy analogs of CP-47,497 (n = 0 to 7) and 1-deoxy analogs of CP-55,940 (n = 0 to 7).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
206.6 °F
flash_point_c
97 °C
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel non-symmetric nickel-diimine complexes for the homopolymerization of ethene: Control of branching by catalyst design.
Schmid M, et al.
Zeitschrift fur Naturforschung, B: Chemical Sciences, 57(10), 1141-1146 (2002)
William F Bailey et al.
The Journal of organic chemistry, 71(7), 2825-2828 (2006-03-25)
The outcome of reactions of 1-bromo-4-tert-butylbenzene (1), a representative aryl bromide, with n-BuLi or t-BuLi at 0 degrees C in a variety of solvent systems has been investigated. The products of reactions of 1 with n-BuLi vary significantly with changes
John W Huffman et al.
Bioorganic & medicinal chemistry, 16(1), 322-335 (2007-10-09)
A series of 1-deoxy analogs of CP-47,497 (8 and 13, n=0-7) and 1-deoxy analogs of CP-55,940 (9, n=0-7) have been synthesized and their affinities for the cannabinoid CB(1) and CB(2) receptors have been determined. Although the majority of these compounds
Xiao Yan et al.
Physical chemistry chemical physics : PCCP, 22(11), 6222-6230 (2020-03-05)
Unveiling the reaction mechanism is significant for developing high-performance catalysts. In this paper, a series of precisely controlled PdxM147-x (M = Cu, Pt, Au, Rh, Ru) dendrimer encapsulated nanoparticles (DENs) has been successfully synthesized. The mechanisms of PdxM147-x as catalysts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service