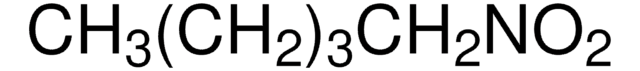About This Item
Recommended Products
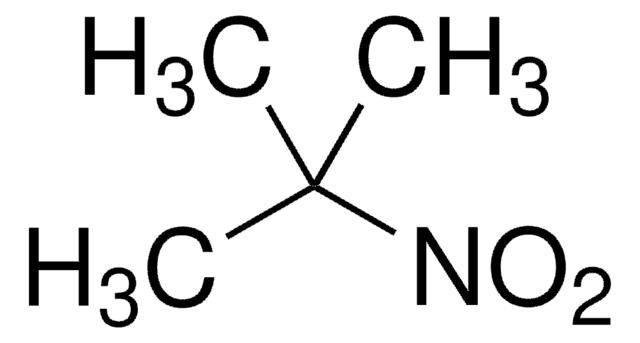
assay
98%
form
liquid
refractive index
n20/D 1.410 (lit.)
bp
152-153 °C (lit.)
mp
−81 °C (lit.)
density
0.973 g/mL at 25 °C (lit.)
functional group
amine
nitro
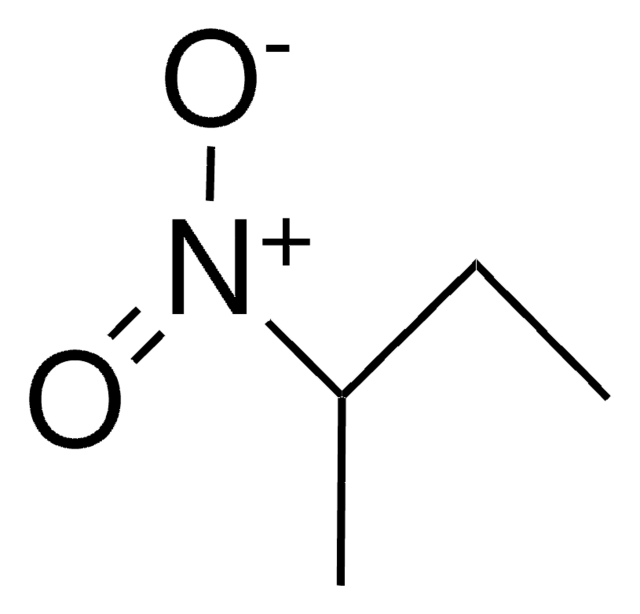
SMILES string
CCCC[N+]([O-])=O
InChI
1S/C4H9NO2/c1-2-3-4-5(6)7/h2-4H2,1H3
InChI key
NALZTFARIYUCBY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
A shortened review of fundamentals of Porous Graphitic Carbon (PGC) materials. What they are and how do they work as a stationary phase material in HPLC?.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service