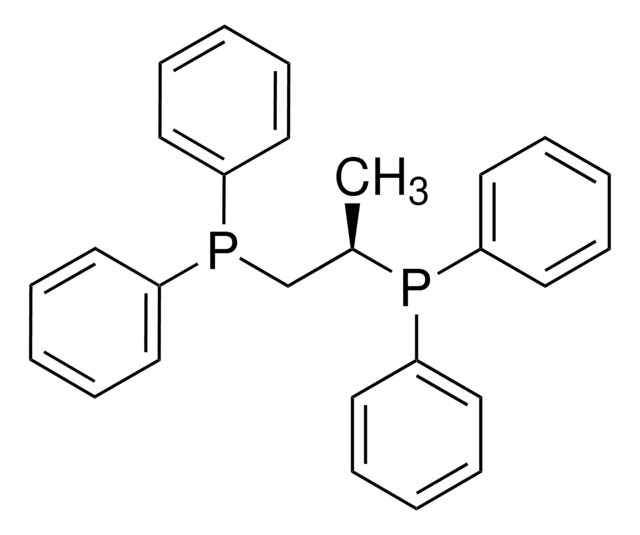
262048
1,3-Bis(diphenylphosphino)propane
97%
Synonym(s):
dppp
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Carbonylations
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
63-65 °C (lit.)
functional group
phosphine
SMILES string
C(CP(c1ccccc1)c2ccccc2)CP(c3ccccc3)c4ccccc4
InChI
1S/C27H26P2/c1-5-14-24(15-6-1)28(25-16-7-2-8-17-25)22-13-23-29(26-18-9-3-10-19-26)27-20-11-4-12-21-27/h1-12,14-21H,13,22-23H2
InChI key
LVEYOSJUKRVCCF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Nanoclusterzyme Dual Colorimetric Sensings: The study explored the utility of 1,3-Bis(diphenylphosphino)propane in creating nanoclusterzymes, demonstrating applications in dual colorimetric sensings. This application suggests significant potential in analytical chemistry and sensor development (Zhao et al., 2023).
- Assembly of Icosahedral Units: Research detailed the use of 1,3-Bis(diphenylphosphino)propane in the stronger assembly of icosahedral units within metal clusters, emphasizing its role in enhancing structural stability and potential applications in nanotechnology (Zhou et al., 2023).
- Synthesis and Stability of Gold Clusters: A publication highlighted the synthesis and evaluation of the stability of mixed-diphosphine ligated gold clusters using 1,3-Bis(diphenylphosphino)propane, underlining its critical role in organometallic chemistry and potential industrial applications (Philliber et al., 2022).
- Size-Conversion in Gold Phosphine Clusters: The work investigated core charge density effects on size-conversion from Au(6) P(8) to Au(8) P(8) Cl(2), facilitated by 1,3-Bis(diphenylphosphino)propane, presenting new insights into cluster chemistry and its implications for catalytic and electronic applications (Lv et al., 2020).
- Synthesis of Donor-Acceptor Diblock Copolymer: This study employed 1,3-Bis(diphenylphosphino)propane in sequential polymerizations to synthesize a novel donor-acceptor diblock copolymer, showcasing the versatility of this ligand in polymer chemistry and its potential for creating advanced materials (Ono et al., 2014).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)