278688
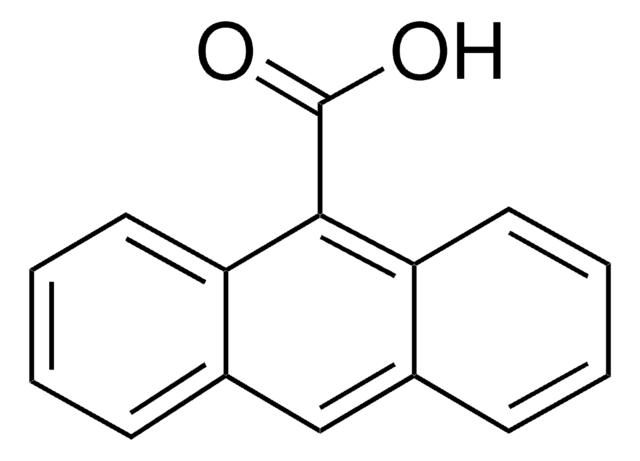
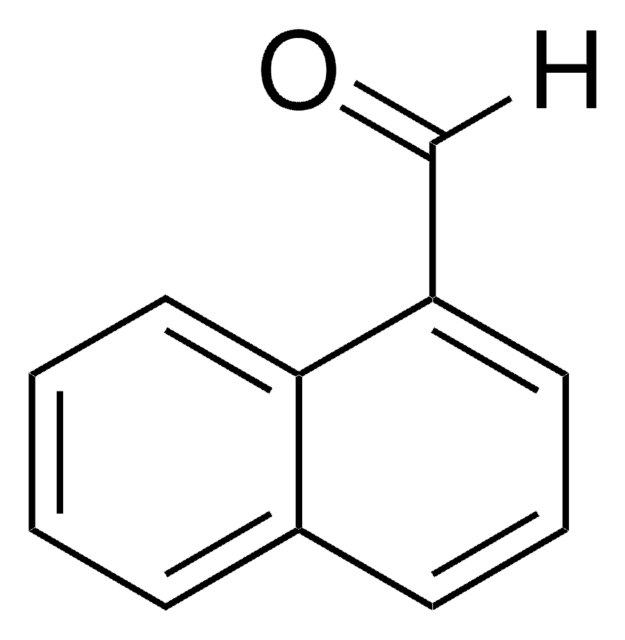
9-Anthracenecarboxaldehyde
97%
Synonym(s):
9-Anthraldehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C15H10O
CAS Number:
Molecular Weight:
206.24
Beilstein/REAXYS Number:
639167
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
103-105 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1c2ccccc2cc3ccccc13
InChI
1S/C15H10O/c16-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)15/h1-10H
InChI key
YMNKUHIVVMFOFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The Diels-Alder reaction of 9-anthracenecarboxaldehyde with benzenediazonium-2-carboxylate was studied.
Application
9-Anthracenecarboxaldehyde has been used in the synthesis of:
- new asymmetrical tridentate Schiff base ligands
- 2-(9-anthrylmethyl-ideneamino)-4-methyl-phenol, novel Schiff base via condensation with 2-amino-p-cresol
- functionalized ligand, 2-(anthracen-9-ylidene)-4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione via Knoevenangel condensation with the diphosphine ligand 4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Skrollan Stockinger et al.
Beilstein journal of organic chemistry, 9, 1837-1842 (2013-09-26)
A new approach for the investigation of a higher-order reaction by on-column reaction gas chromatography is presented. The reaction and the analytical separation are combined in a single experiment to investigate the Diels-Alder reaction of benzenediazonium-2-carboxylate as a benzyne precursor
Mustafa Şahin et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 103, 400-408 (2013-01-01)
New asymmetrical tridentate Schiff base ligands were synthesized using 1,2-phenylenediamine, 4-methyl-1,2-phenylenediamine, 2-hydroxy-1-napthaldehyde, 9-anthracenecarboxaldehyde. Schiff base ligands and their metal complexes were synthesised and characterized by using FT-IR, (1)H NMR, (13)C NMR, UV-Vis, XRD, ESR, elemental analysis and fluorescence studies. The
Knoevenagel condensation of the diphosphine ligand 4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione with 9-anthracenecarboxaldehyde: Synthesis of the second-generation ligand 2-(anthracen-9-ylidene)-4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione.
Watson WH, et al.
Journal of Chemical Crystallography, 36(11), 715-722 (2006)
K Tømmeraas et al.
Carbohydrate research, 336(4), 291-296 (2001-12-01)
Chitosans with chemical composition ranging from a fraction of N-acetylated units (F(A)) of 0.01 to 0.61 were used to prepare fluorescence labelled chitosans by reductive amination with 9-anthraldehyde. Fluorescent chitosans with a low theoretical degree of substitution (DS, 0.001-1%) were
Amitabha Acharya et al.
ACS nano, 4(7), 4061-4073 (2010-06-05)
This paper deals with the self-assembly of the 1:1 complex of two different amphiphiles, namely, a glucosyl-salicyl-imino conjugate (L) and phenylalanine (Phe), forming nanofibers over a period of time through pi...pi interactions. Significant enhancement observed in the fluorescence intensity of
Articles
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service