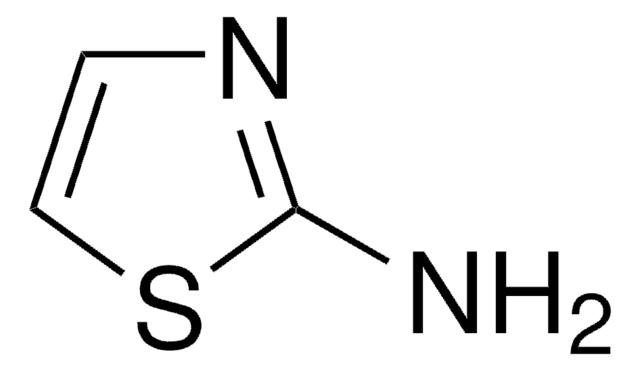
280143
2-Amino-α-(methoxyimino)-4-thiazoleacetic acid, predominantly syn
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7N3O3S
CAS Number:
Molecular Weight:
201.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
192 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
CO\N=C(\C(O)=O)c1csc(N)n1
InChI
1S/C6H7N3O3S/c1-12-9-4(5(10)11)3-2-13-6(7)8-3/h2H,1H3,(H2,7,8)(H,10,11)/b9-4+
InChI key
NLARCUDOUOQRPB-RUDMXATFSA-N
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Total Synthesis of Sodium (3R, 4S)-3-(2-(2-Aminothiazol-4-yl)-(Z)-2-methoxyiminoacetamido)-4-methoxymethyl-2-azetidinone-1-sulfonate from L-Aspartic Acid.
Chung BY, et al.
Bull. Korean Chem. Soc., 13(3), 311-314 (1992)
Total Synthesis of Sodium (3S, 4R)-3-[2-(2-Aminothiazol-4-yl)-(Z)-2-methoxyiminoacetamido]-4-methoxymethyl-2-azetidinone-1-sulfonate from D-Aspartic Acid.
Chung BY, et al.
Bull. Korean Chem. Soc., 13(3), 315-316 (1992)
J V Uri et al.
Acta microbiologica Hungarica, 39(3-4), 317-322 (1992-01-01)
Diazald, a chemical intermediate for the synthesis of biologically active compounds, was found to be a potent in vitro antimicrobial agent against yeasts, yeast-like and filamentous fungi as well as Gram-positive and Gram-negative bacterial strains. Its activity is not inhibited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service