All Photos(1)
About This Item
Empirical Formula (Hill Notation):
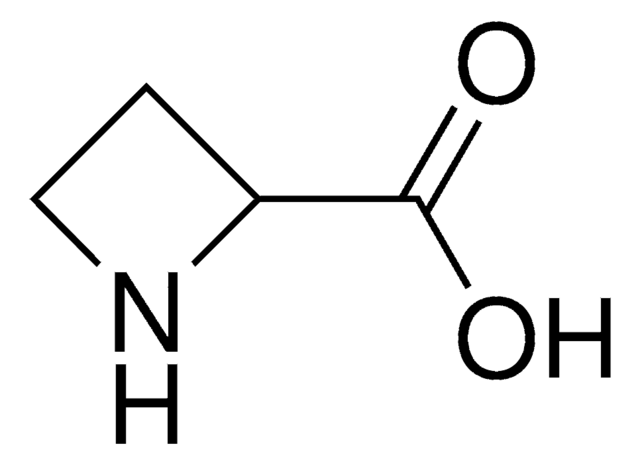
C3H7N
CAS Number:
Molecular Weight:
57.09
Beilstein/REAXYS Number:
102384
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.432 (lit.)
bp
61-62 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CNC1
InChI
1S/C3H7N/c1-2-4-3-1/h4H,1-3H2
InChI key
HONIICLYMWZJFZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phototransformations of azetidine radical cations in freonic matrices under the action of light with λ = 436nm has been investigated. The IR spectrum of azetidine in solid argon matrices has been measured.
Application
Azetidine was employed in:
- a high yielding palladium-catalyzed cross-coupling raection with aryl bromides
- Ullmann type coupling reaction with iodonitroflourenes
accessory
Product No.
Description
Pricing
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-4.0 °F
flash_point_c
-20 °C
ppe
Faceshields, Gloves, Goggles
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phototransformations of azetidine radical cations stabilized in freonic matrices.
Sorokin ID, et al.
High Energy Chemistry, 48(3), 180-184 (2014)
Synthesis, 243-243 (2007)
Experimental and computational studies of the structure and vibrational spectra of azetidine derivatives.
Thompson CA, et al.
Journal of Molecular Structure, 491(1), 67-80 (1999)
Alan P Kozikowski et al.
ChemMedChem, 4(8), 1279-1291 (2009-07-02)
AMOP-H-OH (sazetidine-A; 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) and some sulfur-bearing analogues were tested for their activities in vitro against human alpha4beta2-, alpha4beta4-, alpha3beta4*- and alpha1*-nicotinic acetylcholine receptors (nAChRs). AMOP-H-OH was also assessed in an antidepressant efficacy model. AMOP-H-OH and some of its analogues have
Asta Žukauskaitė et al.
Amino acids, 41(3), 541-558 (2011-03-23)
A short synthesis of alkyl 2-(bromomethyl)aziridine-2-carboxylates and alkyl 3-bromoazetidine-3-carboxylates was developed involving amination, bromination, and base-induced cyclization of alkyl 2-(bromomethyl)acrylates. The aziridines are the kinetically favored cyclization products and could be transformed into 3-bromoazetidine-3-carboxylic acid derivatives via thermal isomerization. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service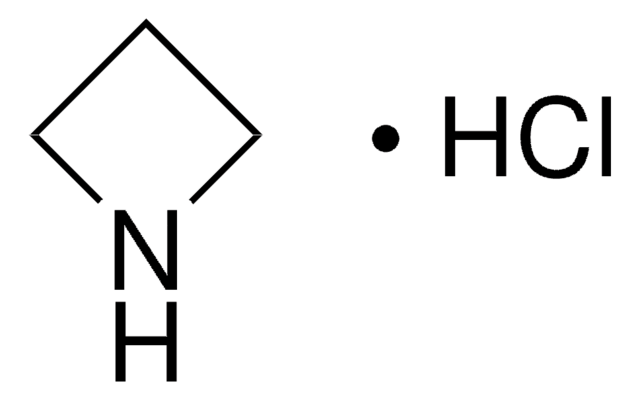
![2-Oxa-6-azaspiro[3.3]heptane](/deepweb/assets/sigmaaldrich/product/structures/391/874/ff74bb51-dd44-4cca-9b3f-3b380ccae360/640/ff74bb51-dd44-4cca-9b3f-3b380ccae360.png)