308080
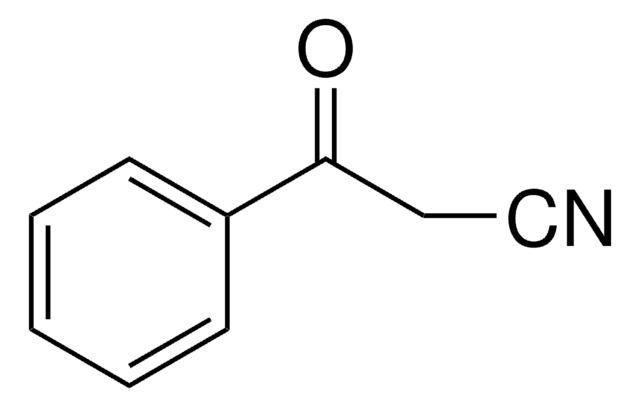
Benzoylnitromethane
98%
Synonym(s):
α-Nitroacetophenone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCH2NO2
CAS Number:
Molecular Weight:
165.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
105-107 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)CC(=O)c1ccccc1
InChI
1S/C8H7NO3/c10-8(6-9(11)12)7-4-2-1-3-5-7/h1-5H,6H2
InChI key
JTWHVBNYYWFXSI-UHFFFAOYSA-N
Related Categories
General description
The kinetics of proton transfer from benzoylnitromethane to various bases was studied.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claude F. Bernasconi et al.
The Journal of organic chemistry, 62(23), 8162-8170 (2001-10-24)
The replacement of a hydrogen in nitromethane and in phenylnitromethane by the PhCO group has a strong acidifying effect, i.e., PhCOCH(2)NO(2), 5, is 5.8, 6.6, and 8.6 pK(a) units more acidic than CH(3)NO(2) in water, 50% DMSO-50% water (v/v), and
Parham Taslimi
Archiv der Pharmazie, 353(11), e2000210-e2000210 (2020-09-03)
In this study, the acetophenone derivatives 1-6 were found to be effective inhibitor molecules for α-glycosidase, human carbonic anhydrases I and II (hCA I/II), and acetylcholinesterase (AChE), with Ki values in the range of 167.98 ± 25.06 to 304.36 ± 65.45 µM for α-glycosidase, 555.76 ± 56.07
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service